

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058977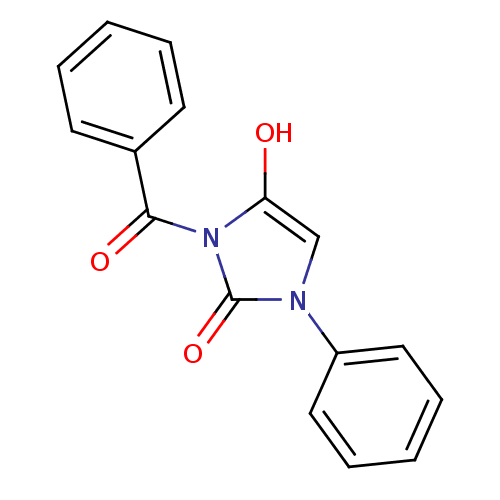 (3-Benzoyl-1-phenyl-imidazolidine-2,4-dione | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50093745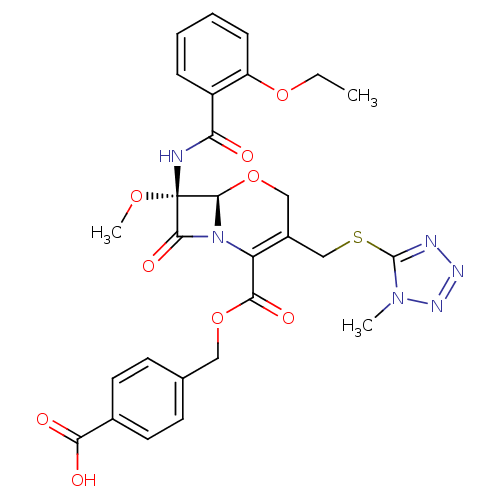 ((6R,7R)-7-(2-Ethoxy-benzoylamino)-7-methoxy-3-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was measured against alpha-chymotrypsin | Bioorg Med Chem Lett 10: 2403-6 (2001) BindingDB Entry DOI: 10.7270/Q23R0S4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059002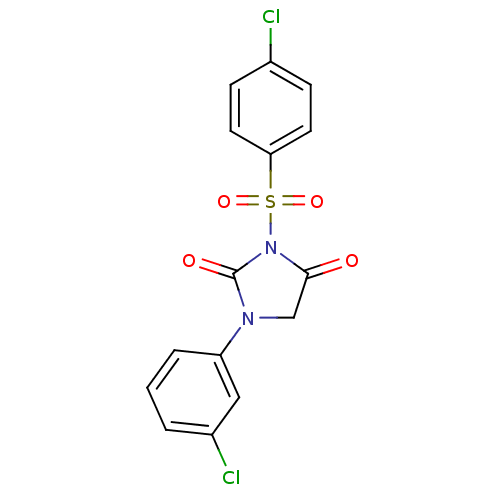 (3-(4-Chloro-benzenesulfonyl)-1-(3-chloro-phenyl)-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059013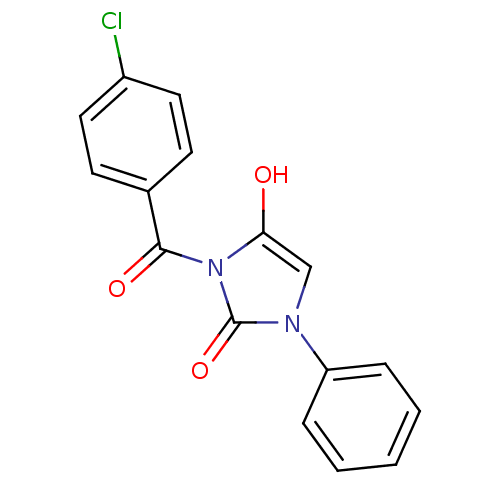 (3-(4-Chloro-benzoyl)-1-phenyl-imidazolidine-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50312532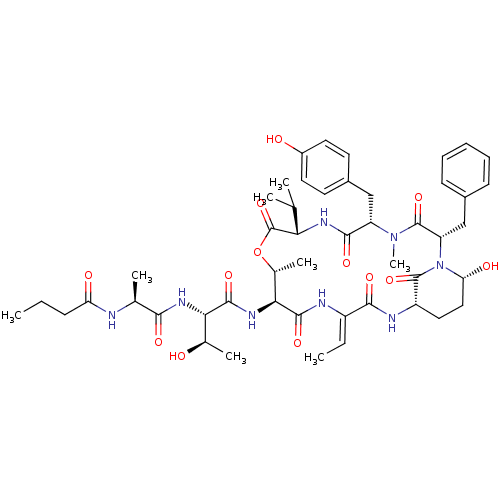 (CHEMBL1077010 | molassamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Smithsonian Marine Station at Ft. Pierce Curated by ChEMBL | Assay Description Inhibition of bovine pancreas alpha-chymotrypsin preincubated for 10 mins by spectrophotometry | J Nat Prod 73: 459-62 (2010) Article DOI: 10.1021/np900603f BindingDB Entry DOI: 10.7270/Q2GH9J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059011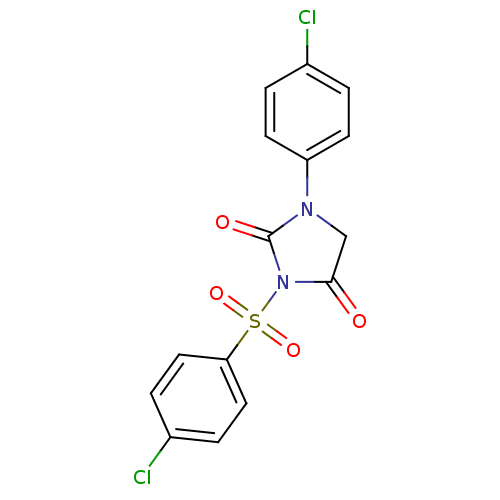 (3-(4-Chloro-benzenesulfonyl)-1-(4-chloro-phenyl)-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118030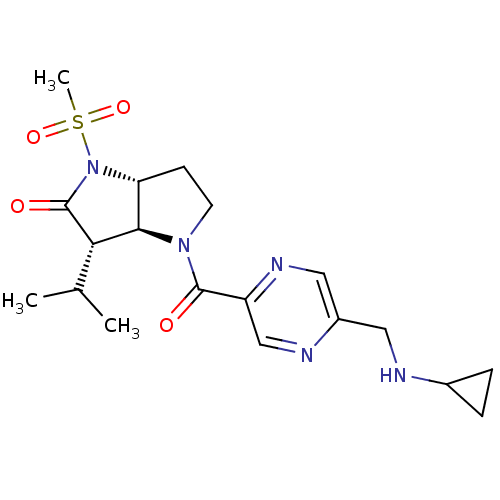 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058975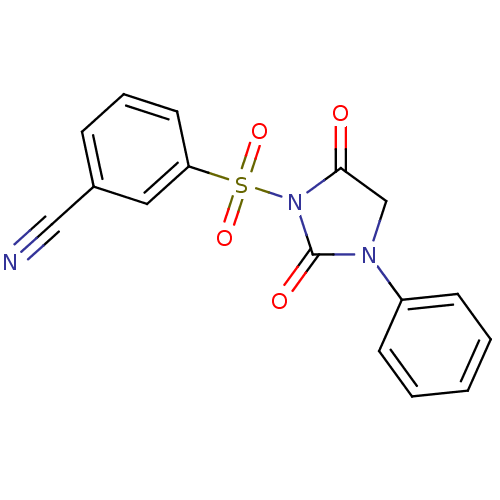 (3-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059012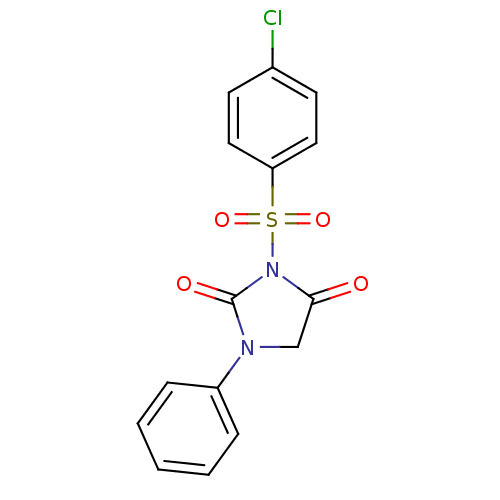 (3-(4-Chloro-benzenesulfonyl)-1-phenyl-imidazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058997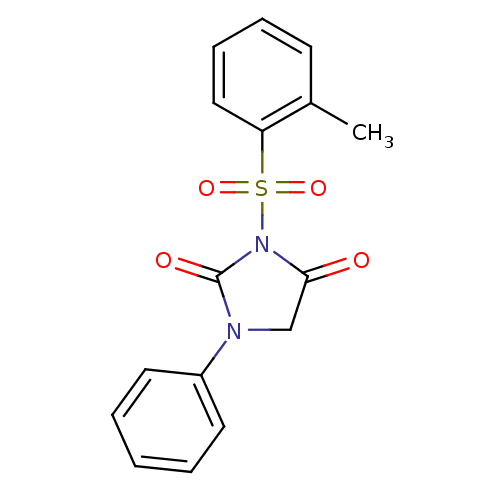 (1-Phenyl-3-(toluene-2-sulfonyl)-imidazolidine-2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50459614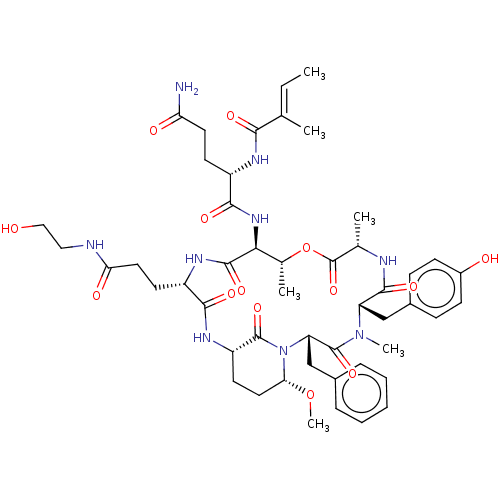 (CHEMBL4212843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of bovine pancreas alpha-chymotrypsin using N-succinyl-Gly-Gly-Phe-p-nitroanilide as substrate incubated for 15 mins followed by substrate... | J Nat Prod 81: 1928-1936 (2018) Article DOI: 10.1021/acs.jnatprod.7b01009 BindingDB Entry DOI: 10.7270/Q2Q81GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058993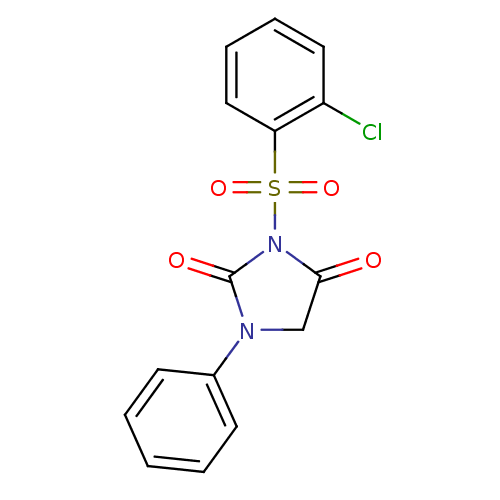 (3-(2-Chloro-benzenesulfonyl)-1-phenyl-imidazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059009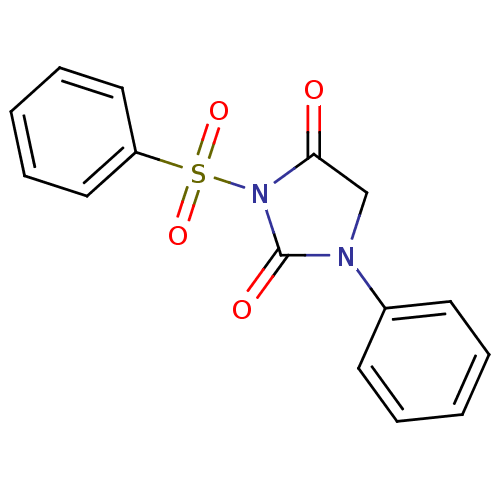 (3-Benzenesulfonyl-1-phenyl-imidazolidine-2,4-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058965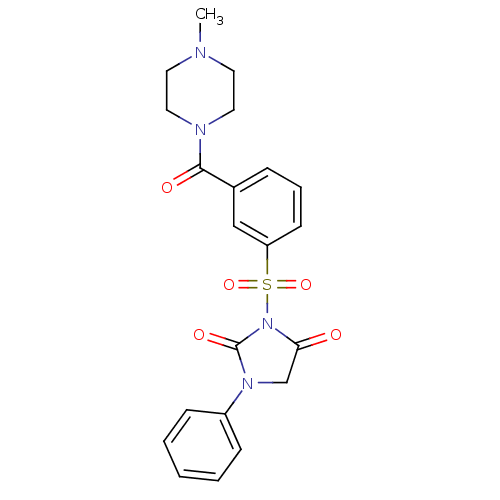 (3-[3-(4-Methyl-piperazine-1-carbonyl)-benzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058967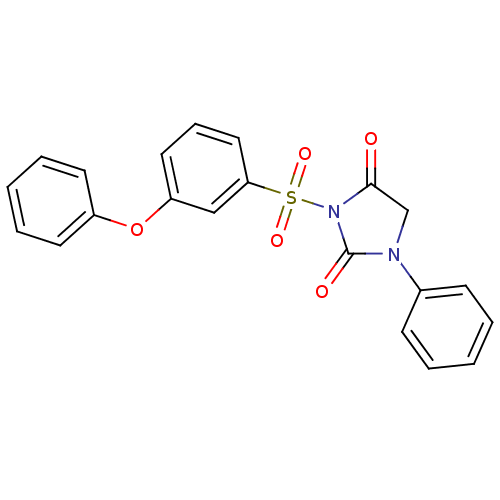 (3-(3-Phenoxy-benzenesulfonyl)-1-phenyl-imidazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50070010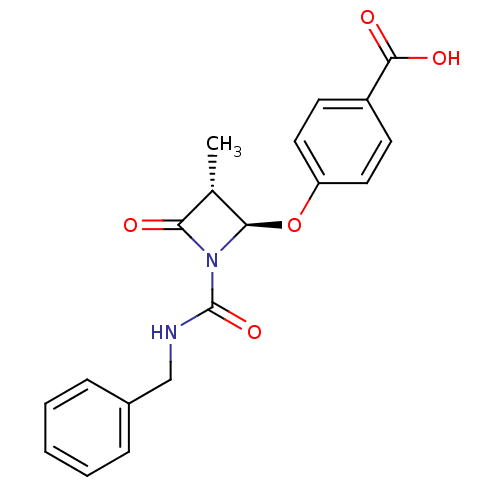 (4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-azetid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit the mammalian Chymotrypsinogen by 50% was determined | Bioorg Med Chem Lett 8: 365-70 (1999) BindingDB Entry DOI: 10.7270/Q2HD7TTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058994 (3-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059007 (4-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058964 (3-(3,4-Dimethyl-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50459616 (CHEMBL4217798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of bovine pancreas alpha-chymotrypsin using N-succinyl-Gly-Gly-Phe-p-nitroanilide as substrate incubated for 15 mins followed by substrate... | J Nat Prod 81: 1928-1936 (2018) Article DOI: 10.1021/acs.jnatprod.7b01009 BindingDB Entry DOI: 10.7270/Q2Q81GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50085342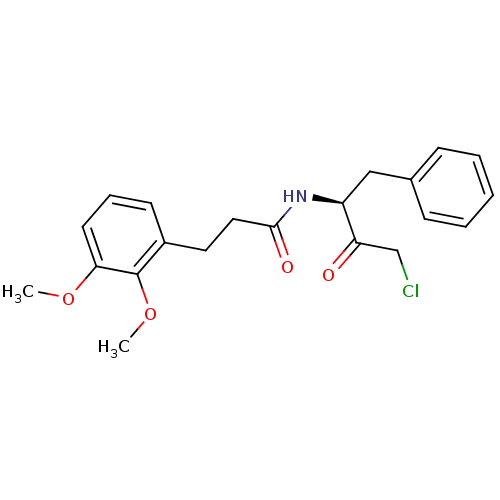 (CHEMBL61059 | N-((S)-1-Benzyl-3-chloro-2-oxo-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity against alpha-chymotrypsin(alpha-CT) | Bioorg Med Chem Lett 10: 199-201 (2000) BindingDB Entry DOI: 10.7270/Q2VM4BGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50065899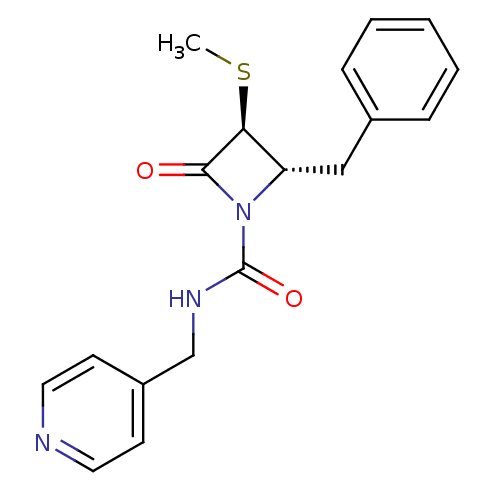 ((2S,3S)-2-Benzyl-3-methylsulfanyl-4-oxo-azetidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Compound was tested for its activity against bovine pancreatic Alpha-chymotrypsin (BPC) | J Med Chem 41: 2882-91 (1998) Article DOI: 10.1021/jm980131z BindingDB Entry DOI: 10.7270/Q2X63M2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50085366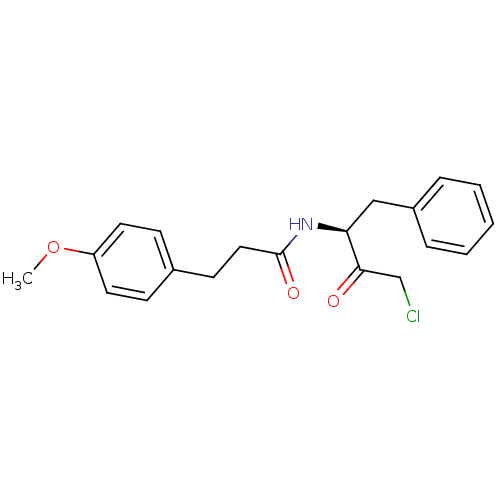 (CHEMBL292834 | N-((S)-1-Benzyl-3-chloro-2-oxo-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity against alpha-chymotrypsin(alpha-CT) | Bioorg Med Chem Lett 10: 199-201 (2000) BindingDB Entry DOI: 10.7270/Q2VM4BGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059005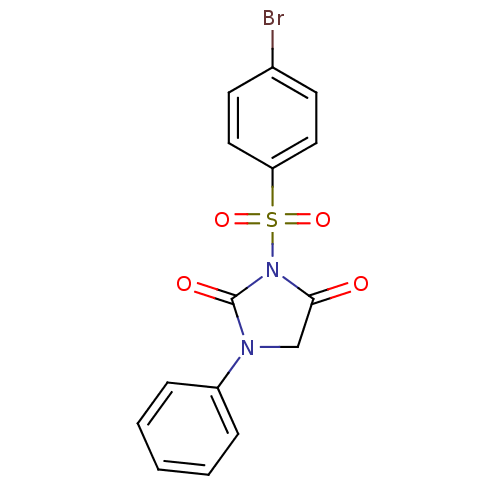 (3-(4-Bromo-benzenesulfonyl)-1-phenyl-imidazolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058998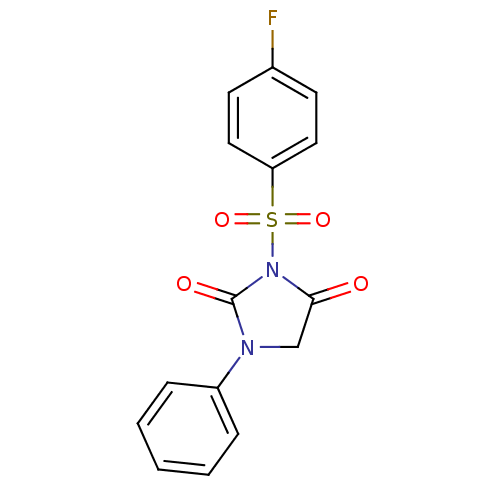 (3-(4-Fluoro-benzenesulfonyl)-1-phenyl-imidazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50085356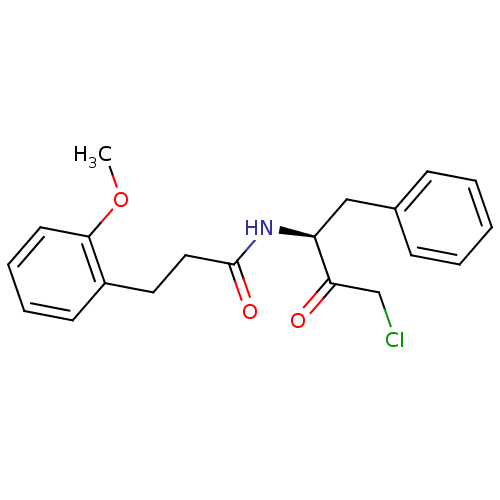 (CHEMBL61933 | N-((S)-1-Benzyl-3-chloro-2-oxo-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity against alpha-chymotrypsin(alpha-CT) | Bioorg Med Chem Lett 10: 199-201 (2000) BindingDB Entry DOI: 10.7270/Q2VM4BGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50118028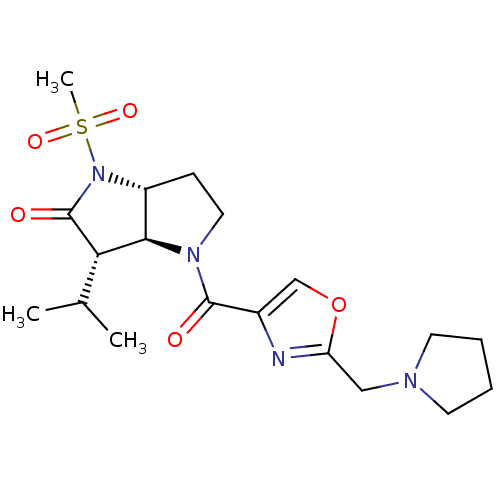 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against Chymotrypsinogen using selectivity assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50199883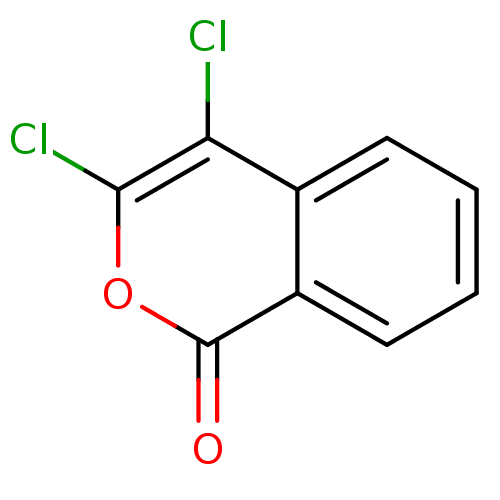 (3,4‐Dichloroisocoumarin (2) | 3,4-Dichloro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine University Duesseldorf Curated by ChEMBL | Assay Description Inhibition of bovine pancreas alpha-chymotrypsin using KSp21 as substrate preincubated for 30 mins followed by substrate addition measured after 50 t... | Bioorg Med Chem Lett 28: 1417-1422 (2018) Article DOI: 10.1016/j.bmcl.2018.02.017 BindingDB Entry DOI: 10.7270/Q2HX1G8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059003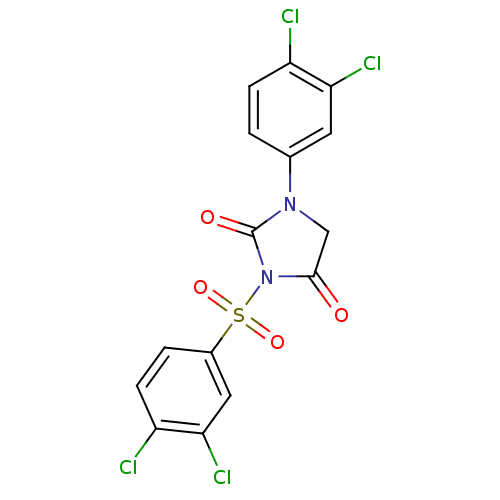 (3-(3,4-Dichloro-benzenesulfonyl)-1-(3,4-dichloro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058983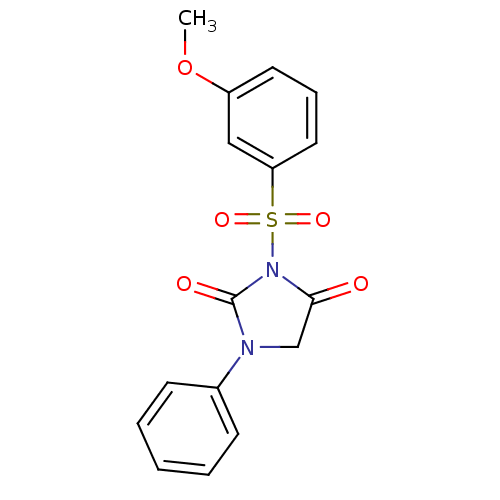 (3-(3-Methoxy-benzenesulfonyl)-1-phenyl-imidazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058973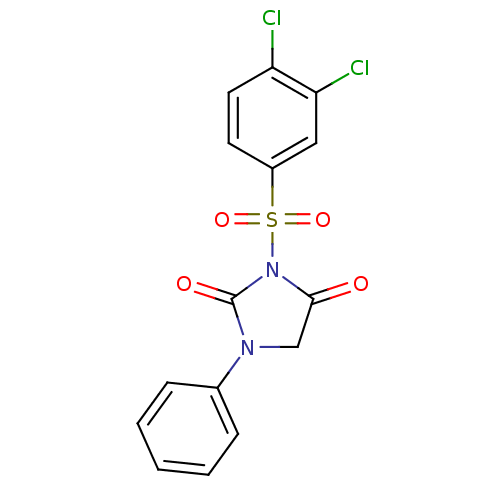 (3-(3,4-Dichloro-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066998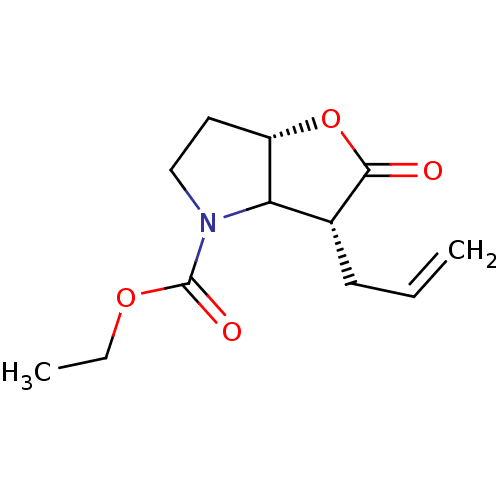 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058996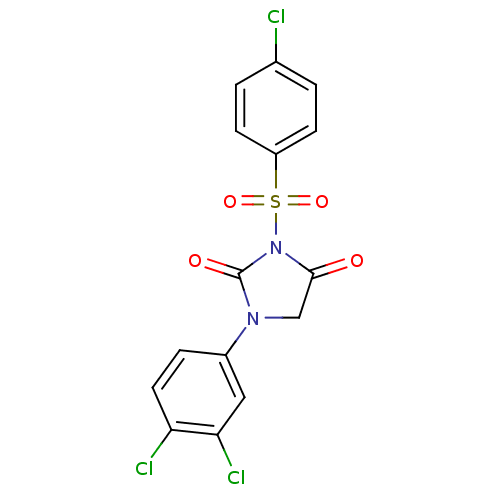 (3-(4-Chloro-benzenesulfonyl)-1-(3,4-dichloro-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50015857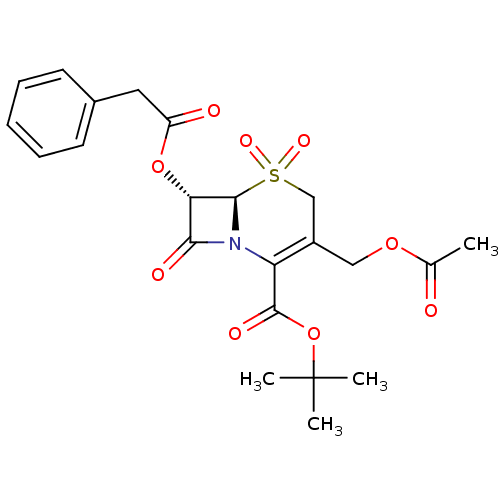 (3-Acetoxymethyl-5,5,8-trioxo-7-phenylacetoxy-5lamb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration which is required to cause 50% inhibition of alpha-chymotrypsin enzyme | J Med Chem 33: 2513-21 (1990) BindingDB Entry DOI: 10.7270/Q2Q81C2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058971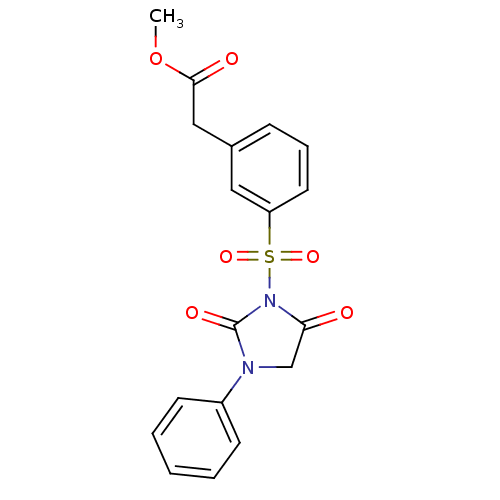 (CHEMBL304338 | [3-(2,5-Dioxo-3-phenyl-imidazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50085346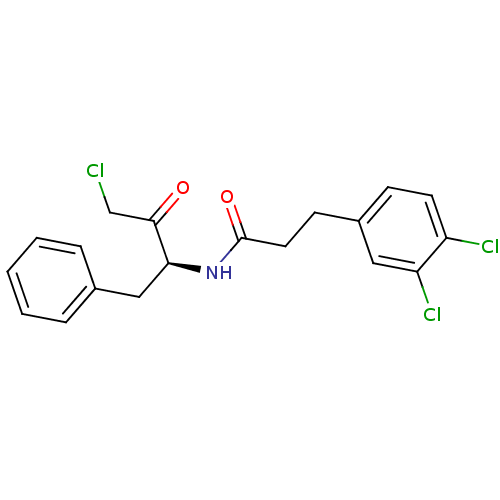 (CHEMBL294290 | N-((S)-1-Benzyl-3-chloro-2-oxo-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity against alpha-chymotrypsin(alpha-CT) | Bioorg Med Chem Lett 10: 199-201 (2000) BindingDB Entry DOI: 10.7270/Q2VM4BGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50066996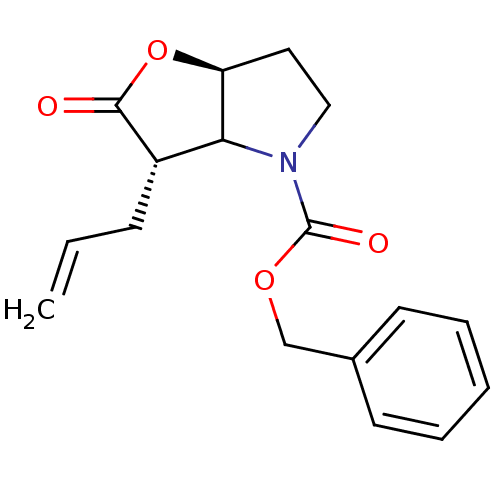 ((3S,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of Chymotrypsinogen with a preincubation time of 15 min | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM26139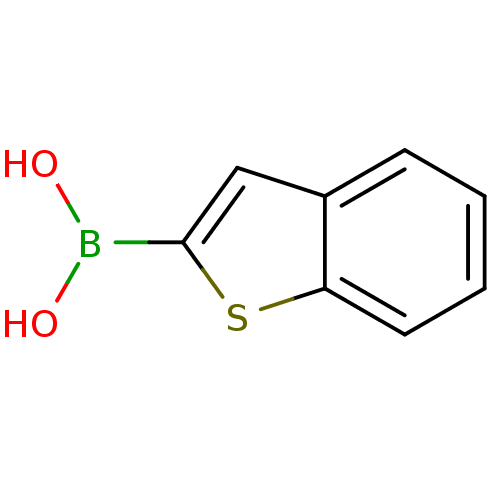 (1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Medical School Curated by ChEMBL | Assay Description Compound was tested for its specificity against alpha-chymotrypsin | J Med Chem 41: 4577-86 (1998) Article DOI: 10.1021/jm980343w BindingDB Entry DOI: 10.7270/Q22N51FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50085361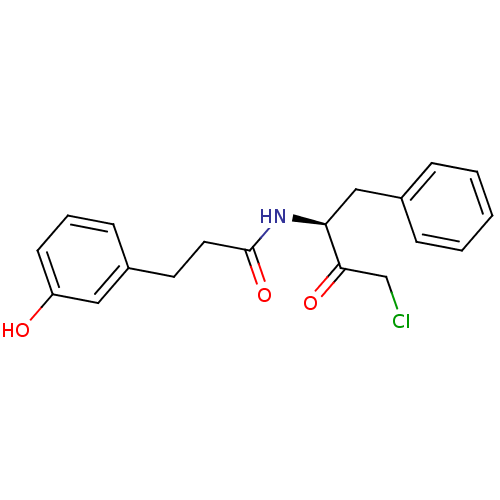 (CHEMBL59998 | N-((S)-1-Benzyl-3-chloro-2-oxo-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory activity against alpha-chymotrypsin(alpha-CT) | Bioorg Med Chem Lett 10: 199-201 (2000) BindingDB Entry DOI: 10.7270/Q2VM4BGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059006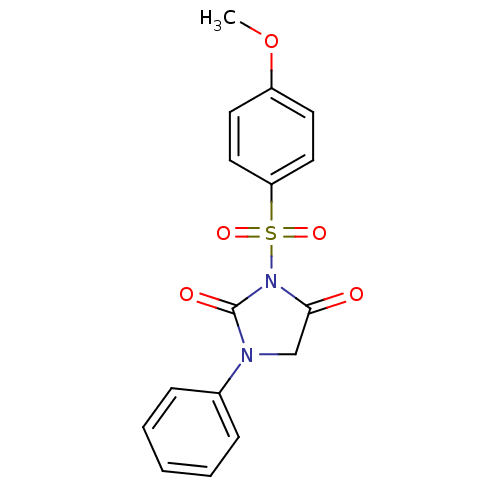 (3-(4-Methoxy-benzenesulfonyl)-1-phenyl-imidazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222139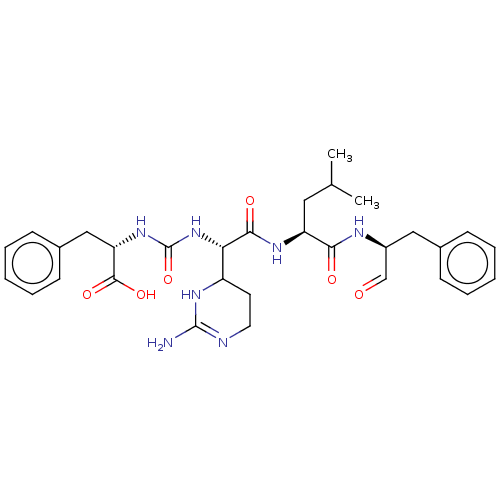 (Chymostatin | US11859014, Compound chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
University of Karachi | Assay Description The α-chymotrypsin inhibition activity was evaluated in 50 mM Tris-HCl buffer pH 7.6 with 10 mM CaCl2. α-Chymotrypsin (bovine pancreas) at ... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50065866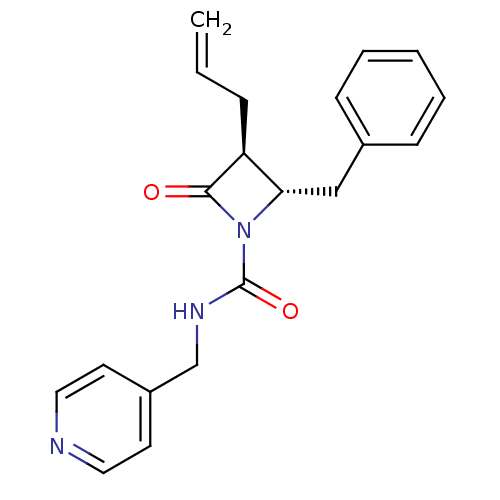 ((2S,3S)-3-Allyl-2-benzyl-4-oxo-azetidine-1-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Compound was tested for its activity against bovine pancreatic Alpha-chymotrypsin (BPC) | J Med Chem 41: 2882-91 (1998) Article DOI: 10.1021/jm980131z BindingDB Entry DOI: 10.7270/Q2X63M2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058995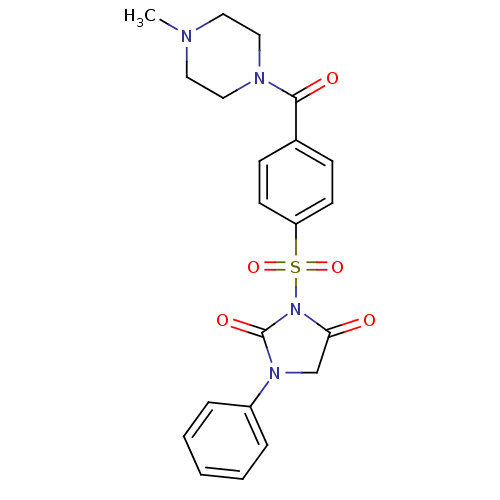 (3-[4-(4-Methyl-piperazine-1-carbonyl)-benzenesulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50289012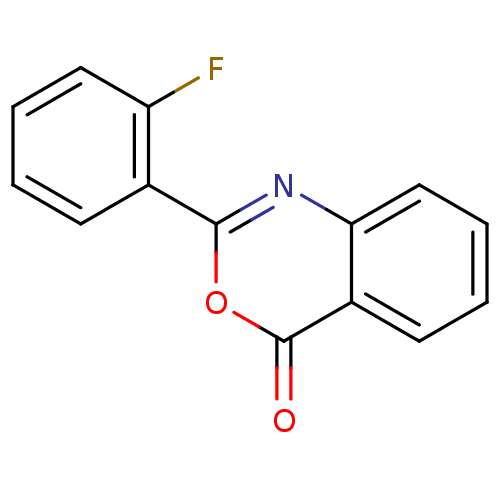 (2-(2-Fluoro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
University of Karachi | Assay Description The α-chymotrypsin inhibition activity was evaluated in 50 mM Tris-HCl buffer pH 7.6 with 10 mM CaCl2. α-Chymotrypsin (bovine pancreas) at ... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50059004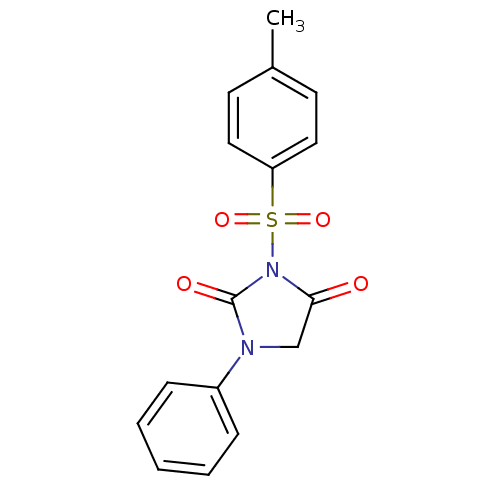 (1-Phenyl-3-(toluene-4-sulfonyl)-imidazolidine-2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058989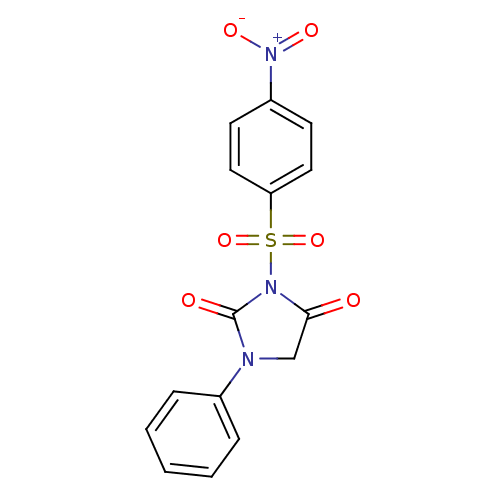 (3-(4-Nitro-benzenesulfonyl)-1-phenyl-imidazolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058966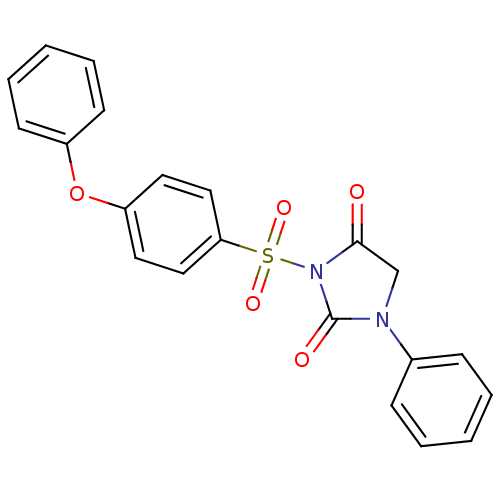 (3-(4-Phenoxy-benzenesulfonyl)-1-phenyl-imidazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50058979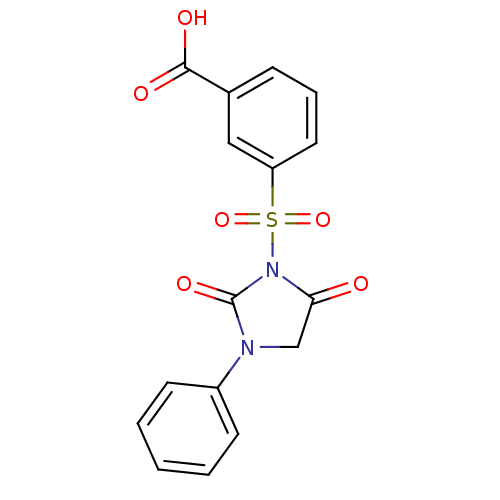 (3-(2,5-Dioxo-3-phenyl-imidazolidine-1-sulfonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against bovine pancreas alpha-chymotrypsin in vitro | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 218 total ) | Next | Last >> |