 Found 64 hits of Enzyme Inhibition Constant Data
Found 64 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160115

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES COC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H16Cl3F2NO3/c1-34-26(33)20-12-19(15-3-5-16(27)6-4-15)24(18-8-7-17(28)11-21(18)29)32-25(20)35-13-14-2-9-22(30)23(31)10-14/h2-12H,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160100
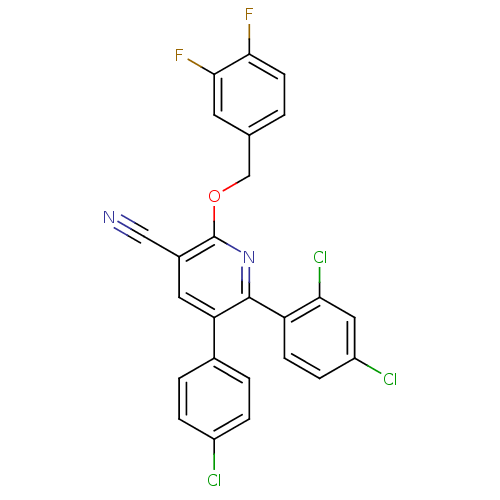
(2-(3,4-difluorobenzyloxy)-5-(4-chlorophenyl)-6-(2,...)Show SMILES Fc1ccc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C25H13Cl3F2N2O/c26-17-4-2-15(3-5-17)20-10-16(12-31)25(33-13-14-1-8-22(29)23(30)9-14)32-24(20)19-7-6-18(27)11-21(19)28/h1-11H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160137
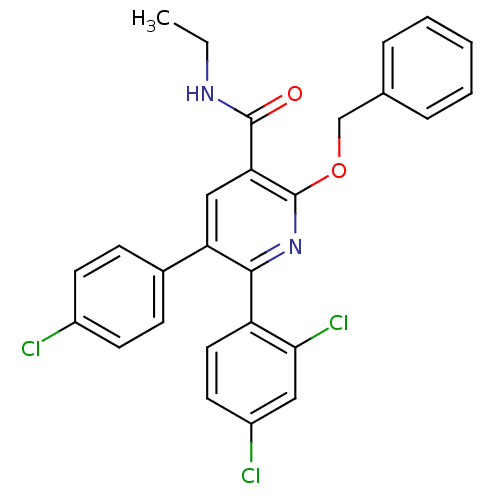
(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES CCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H21Cl3N2O2/c1-2-31-26(33)23-15-22(18-8-10-19(28)11-9-18)25(21-13-12-20(29)14-24(21)30)32-27(23)34-16-17-6-4-3-5-7-17/h3-15H,2,16H2,1H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160131
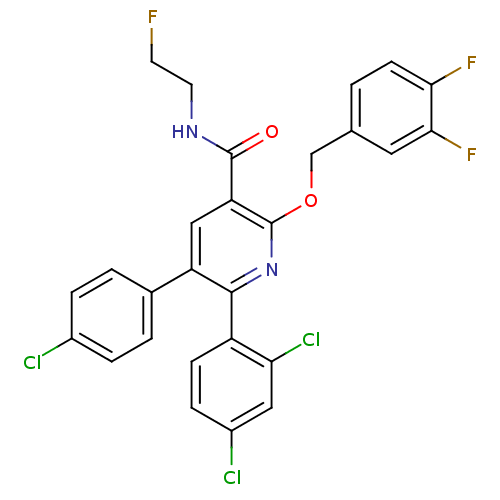
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES FCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H18Cl3F3N2O2/c28-17-4-2-16(3-5-17)20-13-21(26(36)34-10-9-31)27(37-14-15-1-8-23(32)24(33)11-15)35-25(20)19-7-6-18(29)12-22(19)30/h1-8,11-13H,9-10,14H2,(H,34,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160095
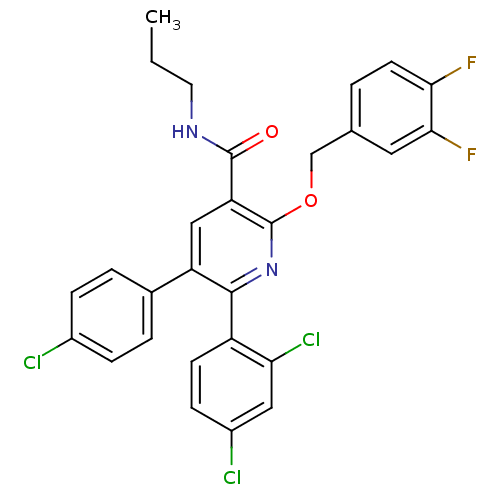
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C28H21Cl3F2N2O2/c1-2-11-34-27(36)22-14-21(17-4-6-18(29)7-5-17)26(20-9-8-19(30)13-23(20)31)35-28(22)37-15-16-3-10-24(32)25(33)12-16/h3-10,12-14H,2,11,15H2,1H3,(H,34,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160143
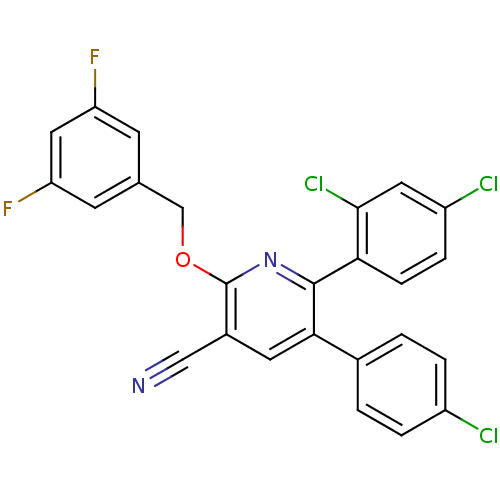
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,5...)Show SMILES Fc1cc(F)cc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C25H13Cl3F2N2O/c26-17-3-1-15(2-4-17)22-9-16(12-31)25(33-13-14-7-19(29)11-20(30)8-14)32-24(22)21-6-5-18(27)10-23(21)28/h1-11H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160132

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CC(C)NC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C28H21Cl3F2N2O2/c1-15(2)34-27(36)22-13-21(17-4-6-18(29)7-5-17)26(20-9-8-19(30)12-23(20)31)35-28(22)37-14-16-3-10-24(32)25(33)11-16/h3-13,15H,14H2,1-2H3,(H,34,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160097

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H19Cl3F2N2O2/c1-2-33-26(35)21-13-20(16-4-6-17(28)7-5-16)25(19-9-8-18(29)12-22(19)30)34-27(21)36-14-15-3-10-23(31)24(32)11-15/h3-13H,2,14H2,1H3,(H,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160145
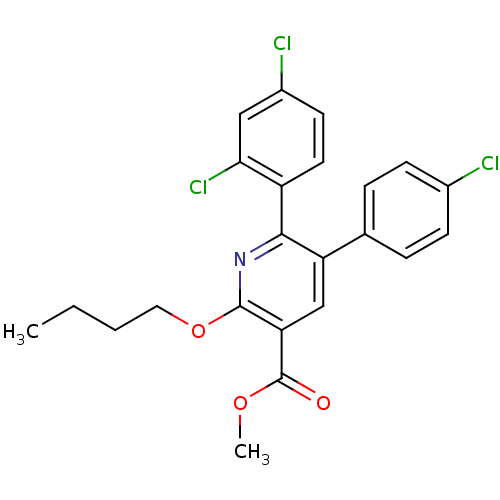
(2-Butoxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CCCCOc1nc(-c2ccc(Cl)cc2Cl)c(cc1C(=O)OC)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H20Cl3NO3/c1-3-4-11-30-22-19(23(28)29-2)13-18(14-5-7-15(24)8-6-14)21(27-22)17-10-9-16(25)12-20(17)26/h5-10,12-13H,3-4,11H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160103
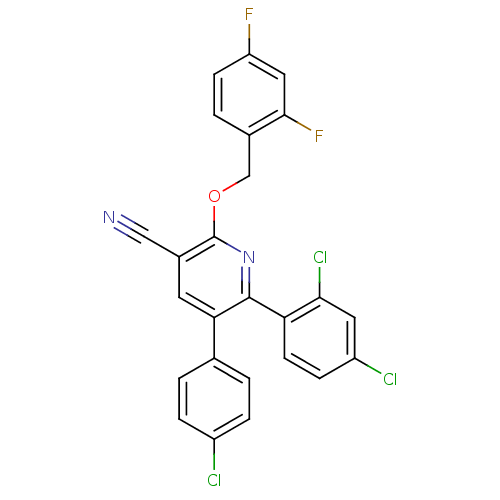
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(2,4...)Show SMILES Fc1ccc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)c(F)c1 Show InChI InChI=1S/C25H13Cl3F2N2O/c26-17-4-1-14(2-5-17)21-9-16(12-31)25(33-13-15-3-7-19(29)11-23(15)30)32-24(21)20-8-6-18(27)10-22(20)28/h1-11H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160136

(5-(4-Chloro-phenyl)-2-cyclohexylmethoxy-6-(2,4-dic...)Show SMILES COC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCC1CCCCC1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H24Cl3NO3/c1-32-26(31)22-14-21(17-7-9-18(27)10-8-17)24(20-12-11-19(28)13-23(20)29)30-25(22)33-15-16-5-3-2-4-6-16/h7-14,16H,2-6,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160099
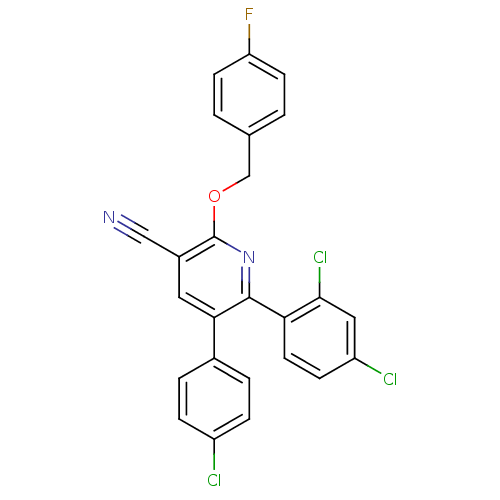
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(4-f...)Show SMILES Fc1ccc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C25H14Cl3FN2O/c26-18-5-3-16(4-6-18)22-11-17(13-30)25(32-14-15-1-8-20(29)9-2-15)31-24(22)21-10-7-19(27)12-23(21)28/h1-12H,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160125

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H17Cl3F2N2O2/c1-32-25(34)20-12-19(15-3-5-16(27)6-4-15)24(18-8-7-17(28)11-21(18)29)33-26(20)35-13-14-2-9-22(30)23(31)10-14/h2-12H,13H2,1H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160108

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(4-t...)Show SMILES FC(F)(F)c1ccc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H14Cl3F3N2O/c27-19-7-3-16(4-8-19)22-11-17(13-33)25(34-24(22)21-10-9-20(28)12-23(21)29)35-14-15-1-5-18(6-2-15)26(30,31)32/h1-12H,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160117

(5-(4-Chloro-phenyl)-2-cyclohexylmethoxy-6-(2,4-dic...)Show SMILES CNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCC1CCCCC1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H25Cl3N2O2/c1-30-25(32)22-14-21(17-7-9-18(27)10-8-17)24(20-12-11-19(28)13-23(20)29)31-26(22)33-15-16-5-3-2-4-6-16/h7-14,16H,2-6,15H2,1H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160113

(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES COC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H18Cl3NO3/c1-32-26(31)22-14-21(17-7-9-18(27)10-8-17)24(20-12-11-19(28)13-23(20)29)30-25(22)33-15-16-5-3-2-4-6-16/h2-14H,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160092
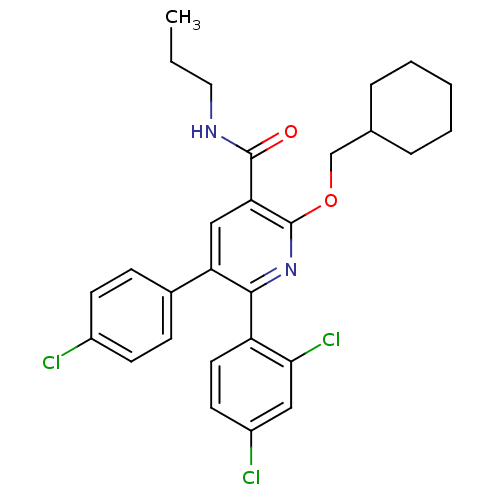
(5-(4-Chloro-phenyl)-2-cyclohexylmethoxy-6-(2,4-dic...)Show SMILES CCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCC1CCCCC1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C28H29Cl3N2O2/c1-2-14-32-27(34)24-16-23(19-8-10-20(29)11-9-19)26(22-13-12-21(30)15-25(22)31)33-28(24)35-17-18-6-4-3-5-7-18/h8-13,15-16,18H,2-7,14,17H2,1H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160133
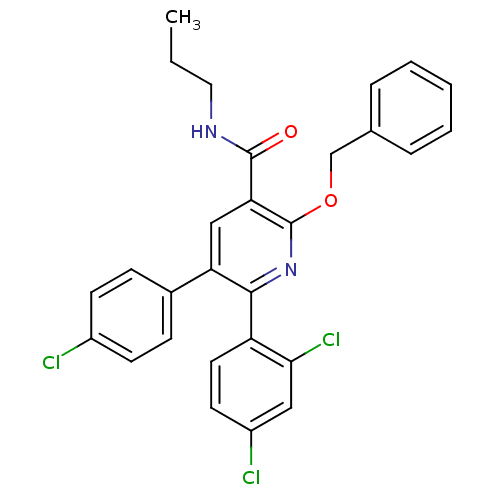
(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES CCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C28H23Cl3N2O2/c1-2-14-32-27(34)24-16-23(19-8-10-20(29)11-9-19)26(22-13-12-21(30)15-25(22)31)33-28(24)35-17-18-6-4-3-5-7-18/h3-13,15-16H,2,14,17H2,1H3,(H,32,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160112

(6-(2,4-Dichloro-phenyl)-2-(3,4-difluoro-benzyloxy)...)Show SMILES Cc1ccc(cc1)-c1cc(C#N)c(OCc2ccc(F)c(F)c2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H16Cl2F2N2O/c1-15-2-5-17(6-3-15)21-11-18(13-31)26(33-14-16-4-9-23(29)24(30)10-16)32-25(21)20-8-7-19(27)12-22(20)28/h2-12H,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160127

(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES CNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H19Cl3N2O2/c1-30-25(32)22-14-21(17-7-9-18(27)10-8-17)24(20-12-11-19(28)13-23(20)29)31-26(22)33-15-16-5-3-2-4-6-16/h2-14H,15H2,1H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160128
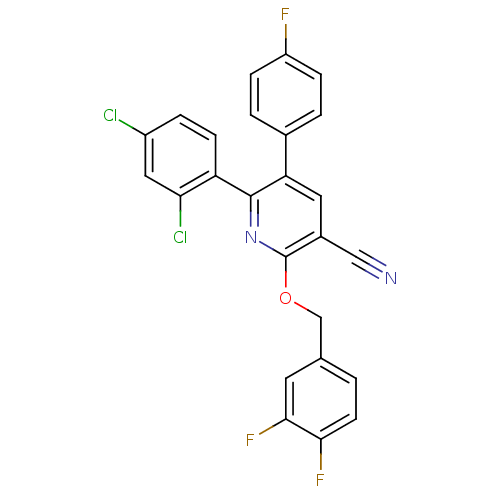
(6-(2,4-Dichloro-phenyl)-2-(3,4-difluoro-benzyloxy)...)Show SMILES Fc1ccc(cc1)-c1cc(C#N)c(OCc2ccc(F)c(F)c2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H13Cl2F3N2O/c26-17-4-7-19(21(27)11-17)24-20(15-2-5-18(28)6-3-15)10-16(12-31)25(32-24)33-13-14-1-8-22(29)23(30)9-14/h1-11H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160138
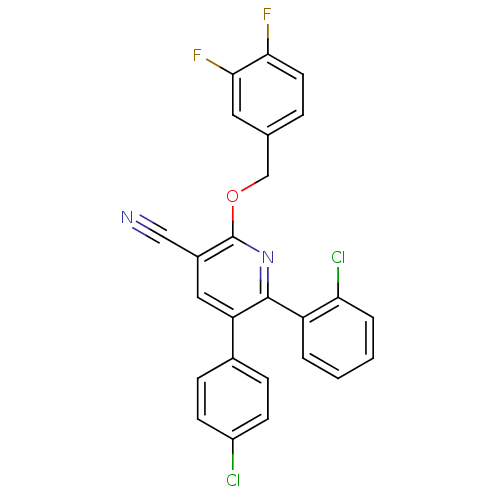
(2-(3,4-difluorobenzyloxy)-6-(2-chlorophenyl)-5-(4-...)Show SMILES Fc1ccc(COc2nc(-c3ccccc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C25H14Cl2F2N2O/c26-18-8-6-16(7-9-18)20-12-17(13-30)25(31-24(20)19-3-1-2-4-21(19)27)32-14-15-5-10-22(28)23(29)11-15/h1-12H,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160107
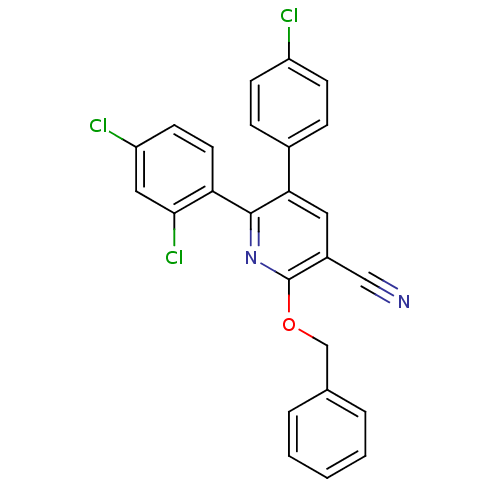
(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES Clc1ccc(cc1)-c1cc(C#N)c(OCc2ccccc2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H15Cl3N2O/c26-19-8-6-17(7-9-19)22-12-18(14-29)25(31-15-16-4-2-1-3-5-16)30-24(22)21-11-10-20(27)13-23(21)28/h1-13H,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160111
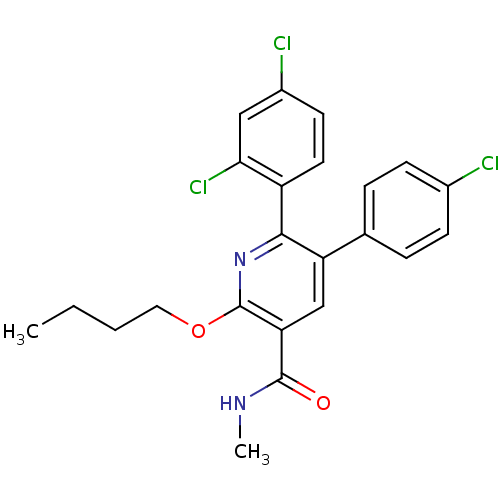
(2-Butoxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CCCCOc1nc(-c2ccc(Cl)cc2Cl)c(cc1C(=O)NC)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H21Cl3N2O2/c1-3-4-11-30-23-19(22(29)27-2)13-18(14-5-7-15(24)8-6-14)21(28-23)17-10-9-16(25)12-20(17)26/h5-10,12-13H,3-4,11H2,1-2H3,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160121
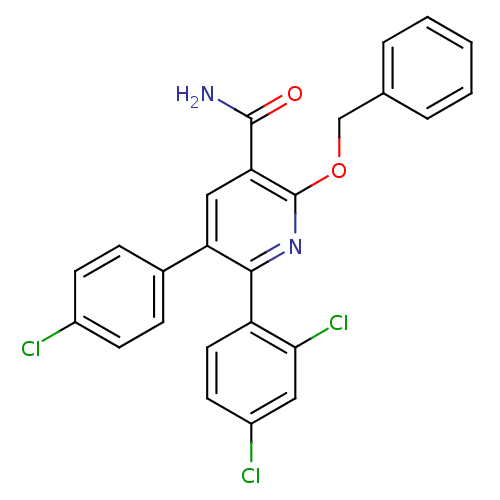
(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES NC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H17Cl3N2O2/c26-17-8-6-16(7-9-17)20-13-21(24(29)31)25(32-14-15-4-2-1-3-5-15)30-23(20)19-11-10-18(27)12-22(19)28/h1-13H,14H2,(H2,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160122
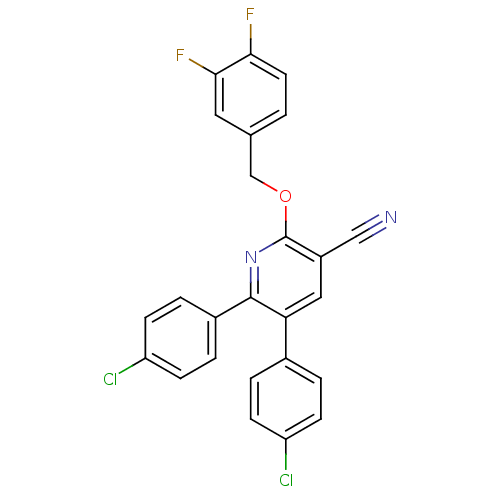
(5,6-Bis-(4-chloro-phenyl)-2-(3,4-difluoro-benzylox...)Show SMILES Fc1ccc(COc2nc(-c3ccc(Cl)cc3)c(cc2C#N)-c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C25H14Cl2F2N2O/c26-19-6-2-16(3-7-19)21-12-18(13-30)25(31-24(21)17-4-8-20(27)9-5-17)32-14-15-1-10-22(28)23(29)11-15/h1-12H,14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160129

(2-Benzyloxy-5-(4-chloro-phenyl)-N-cyclopentyl-6-(2...)Show SMILES Clc1ccc(cc1)-c1cc(C(=O)NC2CCCC2)c(OCc2ccccc2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C30H25Cl3N2O2/c31-21-12-10-20(11-13-21)25-17-26(29(36)34-23-8-4-5-9-23)30(37-18-19-6-2-1-3-7-19)35-28(25)24-15-14-22(32)16-27(24)33/h1-3,6-7,10-17,23H,4-5,8-9,18H2,(H,34,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160124
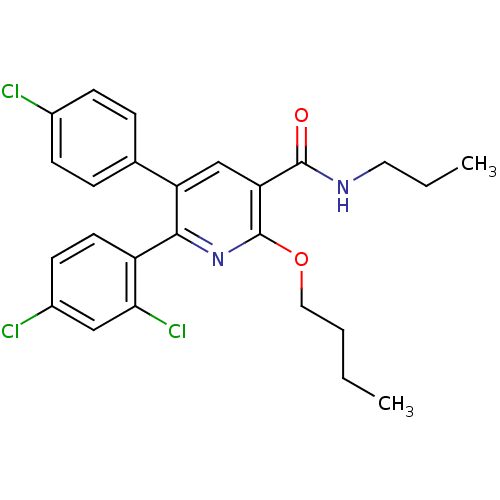
(2-Butoxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CCCCOc1nc(-c2ccc(Cl)cc2Cl)c(cc1C(=O)NCCC)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H25Cl3N2O2/c1-3-5-13-32-25-21(24(31)29-12-4-2)15-20(16-6-8-17(26)9-7-16)23(30-25)19-11-10-18(27)14-22(19)28/h6-11,14-15H,3-5,12-13H2,1-2H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160098
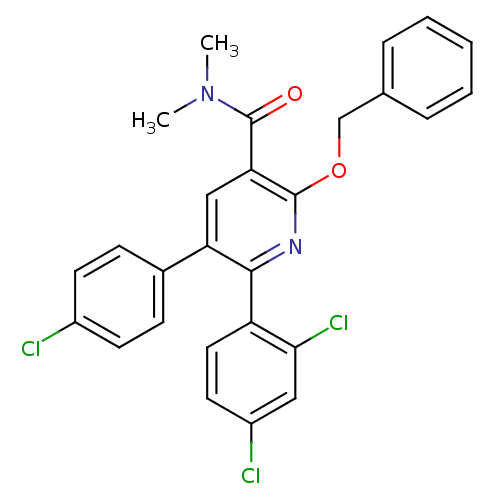
(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES CN(C)C(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H21Cl3N2O2/c1-32(2)27(33)23-15-22(18-8-10-19(28)11-9-18)25(21-13-12-20(29)14-24(21)30)31-26(23)34-16-17-6-4-3-5-7-17/h3-15H,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160116
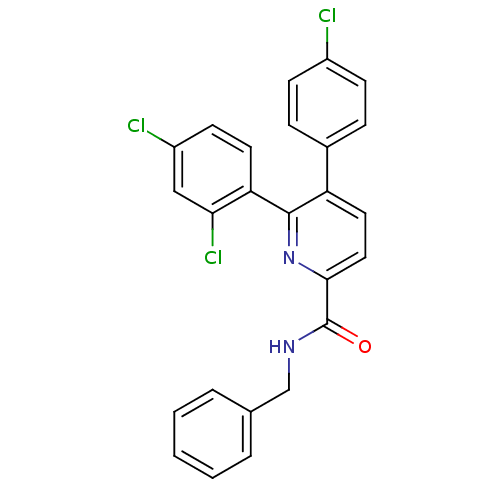
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H17Cl3N2O/c26-18-8-6-17(7-9-18)20-12-13-23(25(31)29-15-16-4-2-1-3-5-16)30-24(20)21-11-10-19(27)14-22(21)28/h1-14H,15H2,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160106

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES CCCC(CCC)NC(=O)c1ccc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H25Cl3N2O/c1-3-5-19(6-4-2)29-25(31)23-14-13-20(16-7-9-17(26)10-8-16)24(30-23)21-12-11-18(27)15-22(21)28/h7-15,19H,3-6H2,1-2H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160105

(2-Butoxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CCCCOc1nc(-c2ccc(Cl)cc2Cl)c(cc1C(N)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19Cl3N2O2/c1-2-3-10-29-22-18(21(26)28)12-17(13-4-6-14(23)7-5-13)20(27-22)16-9-8-15(24)11-19(16)25/h4-9,11-12H,2-3,10H2,1H3,(H2,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160109
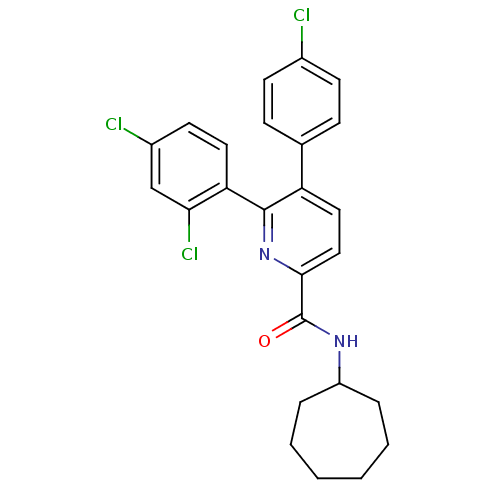
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCCC1 Show InChI InChI=1S/C25H23Cl3N2O/c26-17-9-7-16(8-10-17)20-13-14-23(25(31)29-19-5-3-1-2-4-6-19)30-24(20)21-12-11-18(27)15-22(21)28/h7-15,19H,1-6H2,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160135
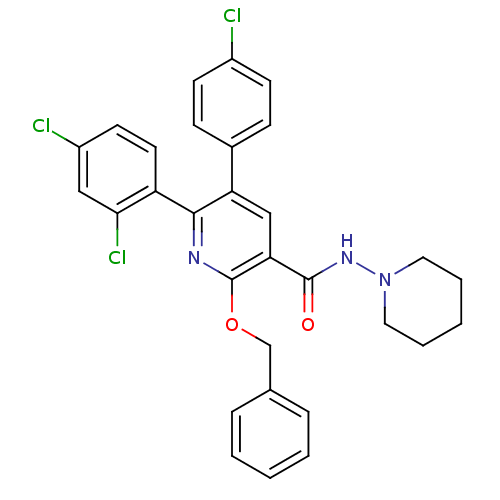
(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES Clc1ccc(cc1)-c1cc(C(=O)NN2CCCCC2)c(OCc2ccccc2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C30H26Cl3N3O2/c31-22-11-9-21(10-12-22)25-18-26(29(37)35-36-15-5-2-6-16-36)30(38-19-20-7-3-1-4-8-20)34-28(25)24-14-13-23(32)17-27(24)33/h1,3-4,7-14,17-18H,2,5-6,15-16,19H2,(H,35,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160104
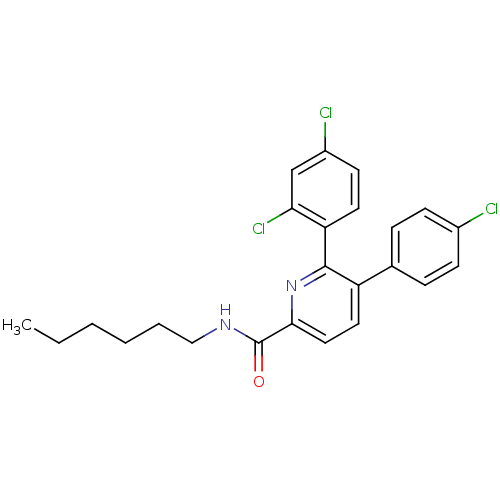
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES CCCCCCNC(=O)c1ccc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H23Cl3N2O/c1-2-3-4-5-14-28-24(30)22-13-12-19(16-6-8-17(25)9-7-16)23(29-22)20-11-10-18(26)15-21(20)27/h6-13,15H,2-5,14H2,1H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160119

(2-Chloro-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1Cl)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C21H16Cl4N2O/c1-2-9-26-21(28)17-11-16(12-3-5-13(22)6-4-12)19(27-20(17)25)15-8-7-14(23)10-18(15)24/h3-8,10-11H,2,9H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160120
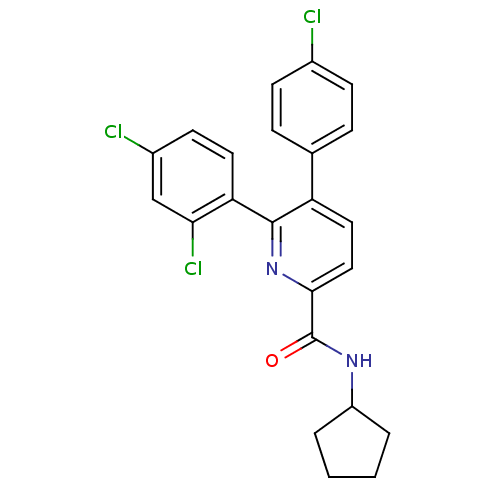
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)NC1CCCC1 Show InChI InChI=1S/C23H19Cl3N2O/c24-15-7-5-14(6-8-15)18-11-12-21(23(29)27-17-3-1-2-4-17)28-22(18)19-10-9-16(25)13-20(19)26/h5-13,17H,1-4H2,(H,27,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160118
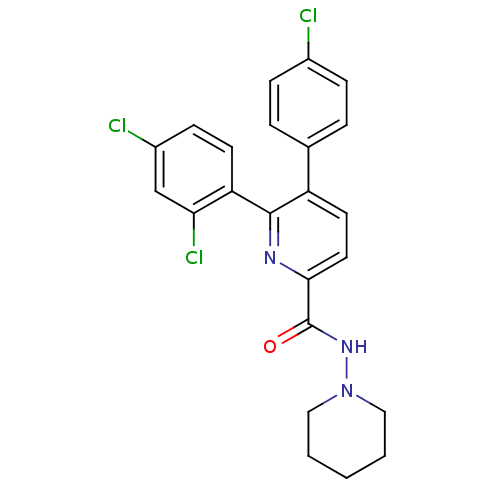
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H20Cl3N3O/c24-16-6-4-15(5-7-16)18-10-11-21(23(30)28-29-12-2-1-3-13-29)27-22(18)19-9-8-17(25)14-20(19)26/h4-11,14H,1-3,12-13H2,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160144

(2-Chloro-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CCCCCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1Cl)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H22Cl4N2O/c1-2-3-4-5-12-29-24(31)20-14-19(15-6-8-16(25)9-7-15)22(30-23(20)28)18-11-10-17(26)13-21(18)27/h6-11,13-14H,2-5,12H2,1H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160102
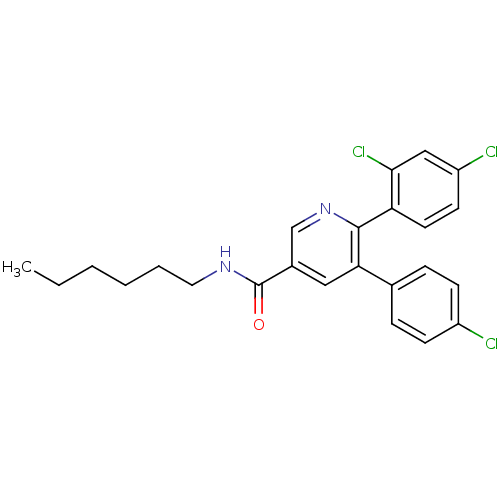
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-N-hexy...)Show SMILES CCCCCCNC(=O)c1cnc(-c2ccc(Cl)cc2Cl)c(c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H23Cl3N2O/c1-2-3-4-5-12-28-24(30)17-13-21(16-6-8-18(25)9-7-16)23(29-15-17)20-11-10-19(26)14-22(20)27/h6-11,13-15H,2-5,12H2,1H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160094
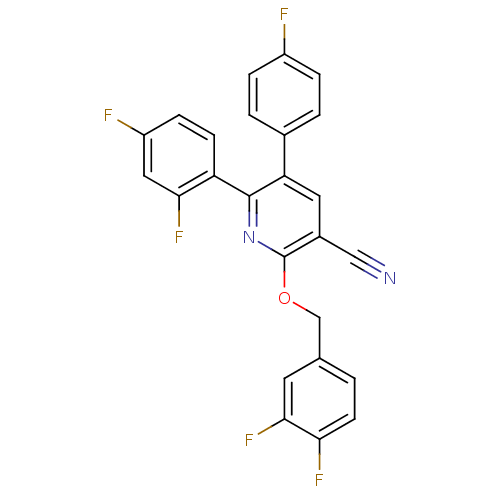
(2-(3,4-Difluoro-benzyloxy)-6-(2,4-difluoro-phenyl)...)Show SMILES Fc1ccc(cc1)-c1cc(C#N)c(OCc2ccc(F)c(F)c2)nc1-c1ccc(F)cc1F Show InChI InChI=1S/C25H13F5N2O/c26-17-4-2-15(3-5-17)20-10-16(12-31)25(33-13-14-1-8-21(28)23(30)9-14)32-24(20)19-7-6-18(27)11-22(19)29/h1-11H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160114

(2-(benzyloxy)-6-(4-chlorophenyl)-5-phenylnicotinon...)Show SMILES Clc1ccc(cc1)-c1nc(OCc2ccccc2)c(cc1-c1ccccc1)C#N Show InChI InChI=1S/C25H17ClN2O/c26-22-13-11-20(12-14-22)24-23(19-9-5-2-6-10-19)15-21(16-27)25(28-24)29-17-18-7-3-1-4-8-18/h1-15H,17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Maximal response (3000 nM) at human cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160123
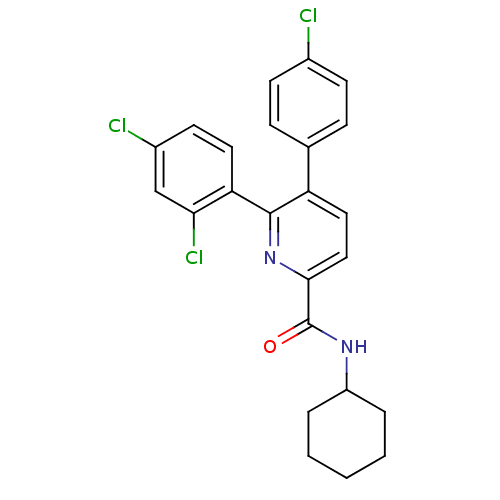
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C24H21Cl3N2O/c25-16-8-6-15(7-9-16)19-12-13-22(24(30)28-18-4-2-1-3-5-18)29-23(19)20-11-10-17(26)14-21(20)27/h6-14,18H,1-5H2,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160096
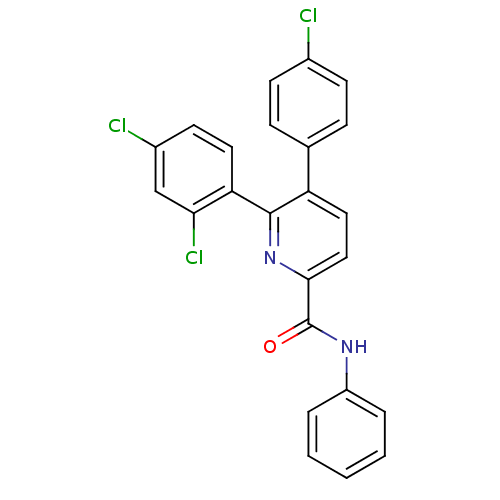
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-pyridi...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)Nc1ccccc1 Show InChI InChI=1S/C24H15Cl3N2O/c25-16-8-6-15(7-9-16)19-12-13-22(24(30)28-18-4-2-1-3-5-18)29-23(19)20-11-10-17(26)14-21(20)27/h1-14H,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160141

(5-(4-Chloro-phenyl)-N-cyclohexyl-6-(2,4-dichloro-p...)Show SMILES Clc1ccc(cc1)-c1cc(cnc1-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C24H21Cl3N2O/c25-17-8-6-15(7-9-17)21-12-16(24(30)29-19-4-2-1-3-5-19)14-28-23(21)20-11-10-18(26)13-22(20)27/h6-14,19H,1-5H2,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160134
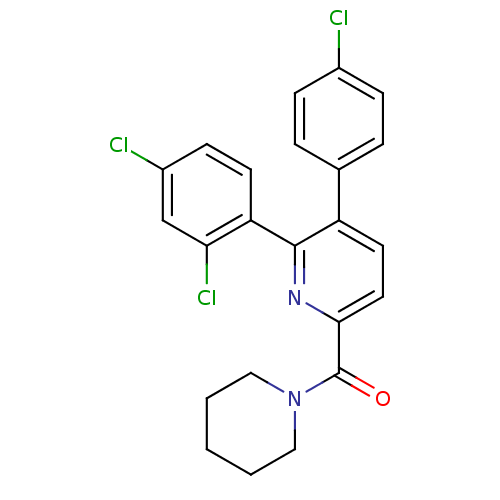
(CHEMBL180507 | [5-(4-Chloro-phenyl)-6-(2,4-dichlor...)Show SMILES Clc1ccc(cc1)-c1ccc(nc1-c1ccc(Cl)cc1Cl)C(=O)N1CCCCC1 Show InChI InChI=1S/C23H19Cl3N2O/c24-16-6-4-15(5-7-16)18-10-11-21(23(29)28-12-2-1-3-13-28)27-22(18)19-9-8-17(25)14-20(19)26/h4-11,14H,1-3,12-13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160140

(2-Chloro-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES Clc1ccc(cc1)-c1cc(C(=O)NN2CCCCC2)c(Cl)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H19Cl4N3O/c24-15-6-4-14(5-7-15)18-13-19(23(31)29-30-10-2-1-3-11-30)22(27)28-21(18)17-9-8-16(25)12-20(17)26/h4-9,12-13H,1-3,10-11H2,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160142

(CHEMBL180154 | [5-(4-Chloro-phenyl)-6-(2,4-dichlor...)Show SMILES Clc1ccc(cc1)-c1cc(cnc1-c1ccc(Cl)cc1Cl)C(=O)N1CCCCC1 Show InChI InChI=1S/C23H19Cl3N2O/c24-17-6-4-15(5-7-17)20-12-16(23(29)28-10-2-1-3-11-28)14-27-22(20)19-9-8-18(25)13-21(19)26/h4-9,12-14H,1-3,10-11H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160110
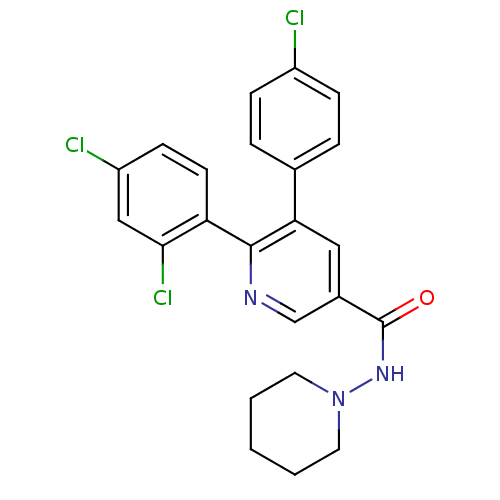
(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-N-pipe...)Show SMILES Clc1ccc(cc1)-c1cc(cnc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H20Cl3N3O/c24-17-6-4-15(5-7-17)20-12-16(23(30)28-29-10-2-1-3-11-29)14-27-22(20)19-9-8-18(25)13-21(19)26/h4-9,12-14H,1-3,10-11H2,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160100

(2-(3,4-difluorobenzyloxy)-5-(4-chlorophenyl)-6-(2,...)Show SMILES Fc1ccc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C25H13Cl3F2N2O/c26-17-4-2-15(3-5-17)20-10-16(12-31)25(33-13-14-1-8-22(29)23(30)9-14)32-24(20)19-7-6-18(27)11-21(19)28/h1-11H,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 2 (hCB2) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160126
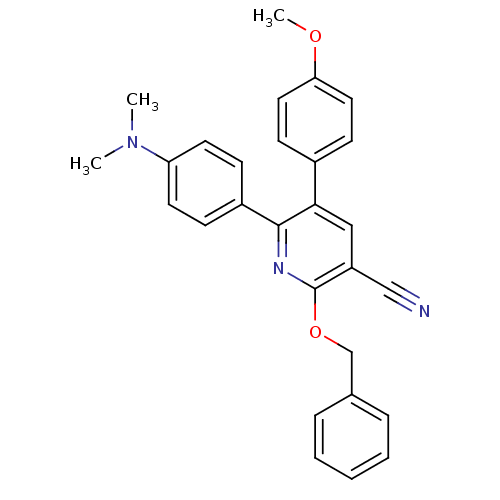
(2-Benzyloxy-6-(4-dimethylamino-phenyl)-5-(4-methox...)Show SMILES COc1ccc(cc1)-c1cc(C#N)c(OCc2ccccc2)nc1-c1ccc(cc1)N(C)C Show InChI InChI=1S/C28H25N3O2/c1-31(2)24-13-9-22(10-14-24)27-26(21-11-15-25(32-3)16-12-21)17-23(18-29)28(30-27)33-19-20-7-5-4-6-8-20/h4-17H,19H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160095

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C28H21Cl3F2N2O2/c1-2-11-34-27(36)22-14-21(17-4-6-18(29)7-5-17)26(20-9-8-19(30)13-23(20)31)35-28(22)37-15-16-3-10-24(32)25(33)12-16/h3-10,12-14H,2,11,15H2,1H3,(H,34,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 815 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 2 (hCB2) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160097

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H19Cl3F2N2O2/c1-2-33-26(35)21-13-20(16-4-6-17(28)7-5-16)25(19-9-8-18(29)12-22(19)30)34-27(21)36-14-15-3-10-23(31)24(32)11-15/h3-13H,2,14H2,1H3,(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 2 (hCB2) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160093
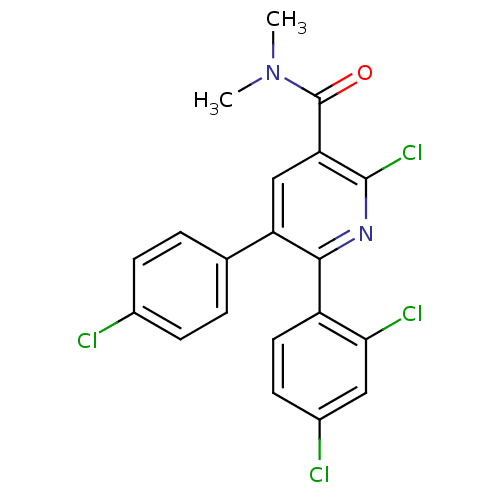
(2-Chloro-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES CN(C)C(=O)c1cc(-c2ccc(Cl)cc2)c(nc1Cl)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H14Cl4N2O/c1-26(2)20(27)16-10-15(11-3-5-12(21)6-4-11)18(25-19(16)24)14-8-7-13(22)9-17(14)23/h3-10H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50160107

(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES Clc1ccc(cc1)-c1cc(C#N)c(OCc2ccccc2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H15Cl3N2O/c26-19-8-6-17(7-9-19)22-12-18(14-29)25(31-15-16-4-2-1-3-5-16)30-24(22)21-11-10-20(27)13-23(21)28/h1-13H,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 2 (hCB2) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160130
(1-Benzyl-5-(4-chloro-phenyl)-6-(2,4-dichloro-pheny...)Show SMILES Clc1ccc(cc1)-c1cc(C#N)c(=O)n(Cc2ccccc2)c1-c1ccc(Cl)cc1Cl |(-6.98,-2.28,;-5.65,-1.5,;-5.65,.04,;-4.32,.8,;-2.97,.04,;-2.97,-1.5,;-4.3,-2.28,;-1.64,.8,;-1.64,2.35,;-.3,3.11,;-.3,4.67,;-.32,6.21,;1.04,2.35,;2.37,3.13,;1.04,.8,;2.37,.04,;3.7,.8,;3.7,2.35,;5.03,3.11,;6.37,2.35,;6.37,.8,;5.03,.04,;-.3,.04,;-.3,-1.5,;-1.64,-2.28,;-1.64,-3.82,;-.3,-4.59,;-.3,-6.13,;1.04,-3.82,;1.04,-2.28,;2.37,-1.5,)| Show InChI InChI=1S/C25H15Cl3N2O/c26-19-8-6-17(7-9-19)22-12-18(14-29)25(31)30(15-16-4-2-1-3-5-16)24(22)21-11-10-20(27)13-23(21)28/h1-13H,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160114

(2-(benzyloxy)-6-(4-chlorophenyl)-5-phenylnicotinon...)Show SMILES Clc1ccc(cc1)-c1nc(OCc2ccccc2)c(cc1-c1ccccc1)C#N Show InChI InChI=1S/C25H17ClN2O/c26-22-13-11-20(12-14-22)24-23(19-9-5-2-6-10-19)15-21(16-27)25(28-24)29-17-18-7-3-1-4-8-18/h1-15H,17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160101
(1-Benzyl-6-(4-chloro-phenyl)-2-oxo-5-phenyl-1,2-di...)Show SMILES Clc1ccc(cc1)-c1c(cc(C#N)c(=O)n1Cc1ccccc1)-c1ccccc1 Show InChI InChI=1S/C25H17ClN2O/c26-22-13-11-20(12-14-22)24-23(19-9-5-2-6-10-19)15-21(16-27)25(29)28(24)17-18-7-3-1-4-8-18/h1-15H,17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160097

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H19Cl3F2N2O2/c1-2-33-26(35)21-13-20(16-4-6-17(28)7-5-16)25(19-9-8-18(29)12-22(19)30)34-27(21)36-14-15-3-10-23(31)24(32)11-15/h3-13H,2,14H2,1H3,(H,33,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160095

(5-(4-Chloro-phenyl)-6-(2,4-dichloro-phenyl)-2-(3,4...)Show SMILES CCCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccc(F)c(F)c1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C28H21Cl3F2N2O2/c1-2-11-34-27(36)22-14-21(17-4-6-18(29)7-5-17)26(20-9-8-19(30)13-23(20)31)35-28(22)37-15-16-3-10-24(32)25(33)12-16/h3-10,12-14H,2,11,15H2,1H3,(H,34,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160137

(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES CCNC(=O)c1cc(-c2ccc(Cl)cc2)c(nc1OCc1ccccc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C27H21Cl3N2O2/c1-2-31-26(33)23-15-22(18-8-10-19(28)11-9-18)25(21-13-12-20(29)14-24(21)30)32-27(23)34-16-17-6-4-3-5-7-17/h3-15H,2,16H2,1H3,(H,31,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160100

(2-(3,4-difluorobenzyloxy)-5-(4-chlorophenyl)-6-(2,...)Show SMILES Fc1ccc(COc2nc(-c3ccc(Cl)cc3Cl)c(cc2C#N)-c2ccc(Cl)cc2)cc1F Show InChI InChI=1S/C25H13Cl3F2N2O/c26-17-4-2-15(3-5-17)20-10-16(12-31)25(33-13-14-1-8-22(29)23(30)9-14)32-24(20)19-7-6-18(27)11-21(19)28/h1-11H,13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50160107

(2-Benzyloxy-5-(4-chloro-phenyl)-6-(2,4-dichloro-ph...)Show SMILES Clc1ccc(cc1)-c1cc(C#N)c(OCc2ccccc2)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H15Cl3N2O/c26-19-8-6-17(7-9-19)22-12-18(14-29)25(31-15-16-4-2-1-3-5-16)30-24(22)21-11-10-20(27)13-23(21)28/h1-13H,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against human Cannabinoid receptor 1 (hCB1) |
Bioorg Med Chem Lett 15: 645-51 (2005)
Article DOI: 10.1016/j.bmcl.2004.11.031
BindingDB Entry DOI: 10.7270/Q2X929SW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data