 Found 116 hits of Enzyme Inhibition Constant Data
Found 116 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167422

(CHEMBL196462 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2Cc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:45,t:11| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,11,17H,9-10H2,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167413
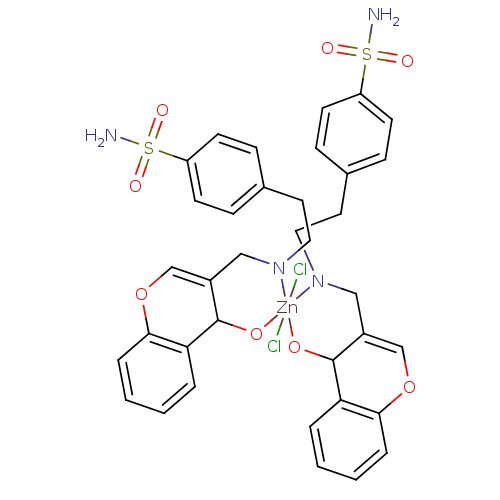
(CHEMBL372750 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(CCN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2CCc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:47,t:12| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,12,18H,9-11H2,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167421
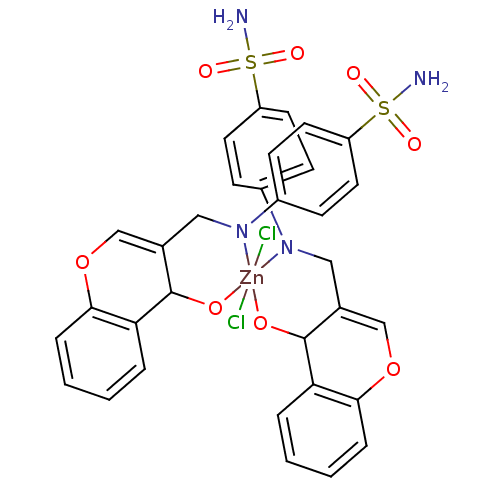
(CHEMBL370172 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(CN1c1ccc(cc1)S(N)(=O)=O)=COc1ccccc21 |c:46,t:13| Show InChI InChI=1S/2C16H14N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-8,10,16H,9H2,(H2,17,20,21);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XI |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167405
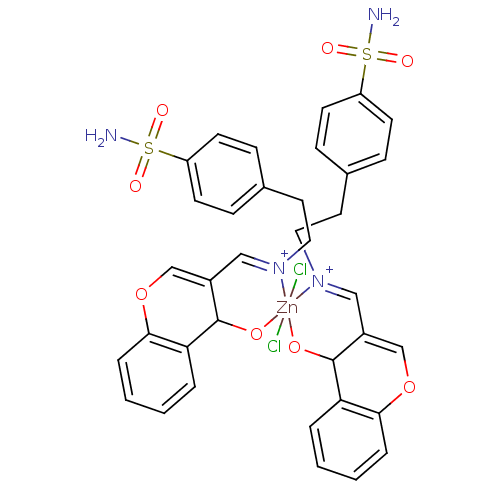
(CHEMBL539686 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CC[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2CCc2ccc(cc2)S(N)(=O)=O)cc1 |c:31,42,t:10,12| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,11-12,18H,9-10H2,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-11+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167415
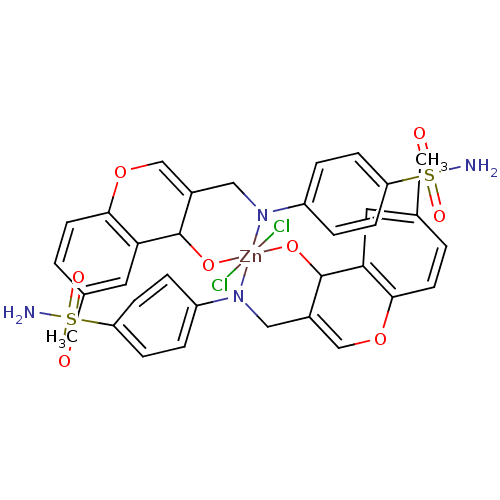
(CHEMBL371127 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4c2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:47,t:6| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-8,10,17H,9H2,1H3,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167414
(CHEMBL274255 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4Cc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:49,t:6| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,11,18H,9-10H2,1H3,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167400
(CHEMBL193958 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4c1ccc(cc1)S(N)(=O)=O |c:33,45,t:6,8| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-10,17H,1H3,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-9+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167405

(CHEMBL539686 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CC[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2CCc2ccc(cc2)S(N)(=O)=O)cc1 |c:31,42,t:10,12| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,11-12,18H,9-10H2,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-11+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167405

(CHEMBL539686 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CC[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2CCc2ccc(cc2)S(N)(=O)=O)cc1 |c:31,42,t:10,12| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,11-12,18H,9-10H2,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-11+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167400

(CHEMBL193958 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4c1ccc(cc1)S(N)(=O)=O |c:33,45,t:6,8| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-10,17H,1H3,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-9+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167413

(CHEMBL372750 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(CCN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2CCc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:47,t:12| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,12,18H,9-11H2,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167400

(CHEMBL193958 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4c1ccc(cc1)S(N)(=O)=O |c:33,45,t:6,8| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-10,17H,1H3,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-9+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167407
(CHEMBL371504 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4Cc1ccc(cc1)S(N)(=O)=O |c:34,46,t:6,8| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,10-11,18H,9H2,1H3,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-10+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167412
(CHEMBL382309 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3CN(CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4CCc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:51,t:6| Show InChI InChI=1S/2C19H20N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10,12,19H,8-9,11H2,1H3,(H2,20,23,24);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167422

(CHEMBL196462 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2Cc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:45,t:11| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,11,17H,9-10H2,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167401
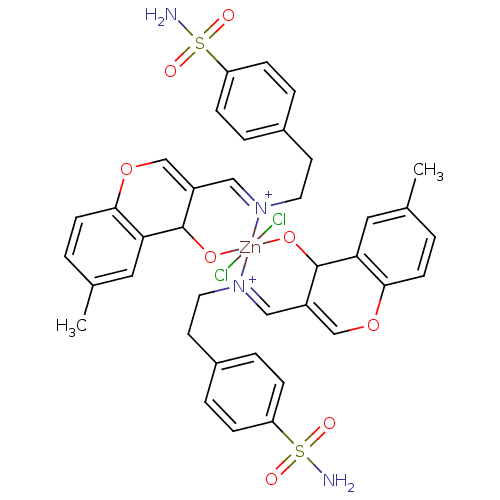
(CHEMBL193432 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4CCc1ccc(cc1)S(N)(=O)=O |c:35,47,t:6,8| Show InChI InChI=1S/2C19H19N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10-12,19H,8-9H2,1H3,(H2,20,23,24);2*1H;/q2*-1;;;+6/p-2/b2*21-11+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167407

(CHEMBL371504 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4Cc1ccc(cc1)S(N)(=O)=O |c:34,46,t:6,8| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,10-11,18H,9H2,1H3,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-10+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167421

(CHEMBL370172 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(CN1c1ccc(cc1)S(N)(=O)=O)=COc1ccccc21 |c:46,t:13| Show InChI InChI=1S/2C16H14N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-8,10,16H,9H2,(H2,17,20,21);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167400

(CHEMBL193958 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4c1ccc(cc1)S(N)(=O)=O |c:33,45,t:6,8| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-10,17H,1H3,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-9+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167413

(CHEMBL372750 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(CCN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2CCc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:47,t:12| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,12,18H,9-11H2,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167412

(CHEMBL382309 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3CN(CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4CCc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:51,t:6| Show InChI InChI=1S/2C19H20N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10,12,19H,8-9,11H2,1H3,(H2,20,23,24);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167401

(CHEMBL193432 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4CCc1ccc(cc1)S(N)(=O)=O |c:35,47,t:6,8| Show InChI InChI=1S/2C19H19N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10-12,19H,8-9H2,1H3,(H2,20,23,24);2*1H;/q2*-1;;;+6/p-2/b2*21-11+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167422

(CHEMBL196462 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2Cc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:45,t:11| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,11,17H,9-10H2,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167405

(CHEMBL539686 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CC[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2CCc2ccc(cc2)S(N)(=O)=O)cc1 |c:31,42,t:10,12| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,11-12,18H,9-10H2,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-11+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167412

(CHEMBL382309 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3CN(CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4CCc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:51,t:6| Show InChI InChI=1S/2C19H20N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10,12,19H,8-9,11H2,1H3,(H2,20,23,24);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167414

(CHEMBL274255 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4Cc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:49,t:6| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,11,18H,9-10H2,1H3,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167407

(CHEMBL371504 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4Cc1ccc(cc1)S(N)(=O)=O |c:34,46,t:6,8| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,10-11,18H,9H2,1H3,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-10+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167408
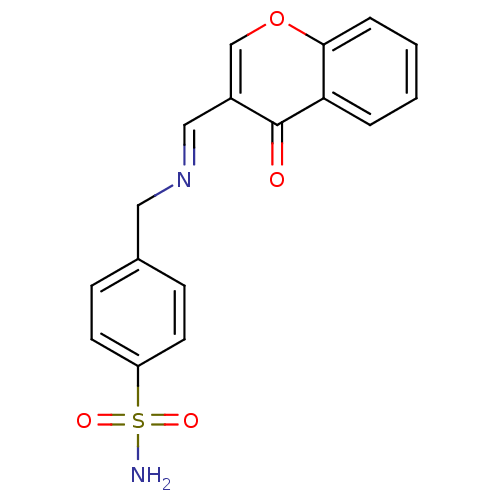
(4-(((4-oxo-4H-chromen-3-yl)methyleneamino)methyl)b...)Show InChI InChI=1S/C17H14N2O4S/c18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20/h1-8,10-11H,9H2,(H2,18,21,22)/b19-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167415

(CHEMBL371127 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4c2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:47,t:6| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-8,10,17H,9H2,1H3,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167413

(CHEMBL372750 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(CCN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2CCc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:47,t:12| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21;;;/h2*1-8,12,18H,9-11H2,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167422

(CHEMBL196462 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(CN2CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(CN2Cc2ccc(cc2)S(N)(=O)=O)=COc2ccccc32)cc1 |c:45,t:11| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,11,17H,9-10H2,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167402
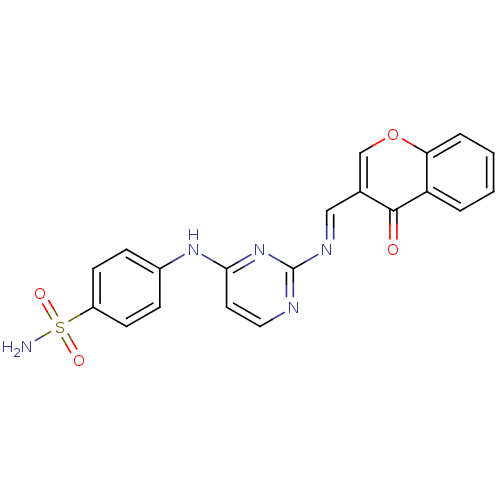
(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)pyrimi...)Show SMILES NS(=O)(=O)c1ccc(Nc2ccnc(\N=C\c3coc4ccccc4c3=O)n2)cc1 Show InChI InChI=1S/C20H15N5O4S/c21-30(27,28)15-7-5-14(6-8-15)24-18-9-10-22-20(25-18)23-11-13-12-29-17-4-2-1-3-16(17)19(13)26/h1-12H,(H2,21,27,28)(H,22,24,25)/b23-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167421

(CHEMBL370172 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(CN1c1ccc(cc1)S(N)(=O)=O)=COc1ccccc21 |c:46,t:13| Show InChI InChI=1S/2C16H14N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-8,10,16H,9H2,(H2,17,20,21);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167401

(CHEMBL193432 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4CCc1ccc(cc1)S(N)(=O)=O |c:35,47,t:6,8| Show InChI InChI=1S/2C19H19N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10-12,19H,8-9H2,1H3,(H2,20,23,24);2*1H;/q2*-1;;;+6/p-2/b2*21-11+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167418

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\c3nccc(Nc4ccc(cc4)S(N)(=O)=O)n3)c(=O)c2c1 Show InChI InChI=1S/C21H17N5O4S/c1-13-2-7-18-17(10-13)20(27)14(12-30-18)11-24-21-23-9-8-19(26-21)25-15-3-5-16(6-4-15)31(22,28)29/h2-12H,1H3,(H2,22,28,29)(H,23,25,26)/b24-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167406
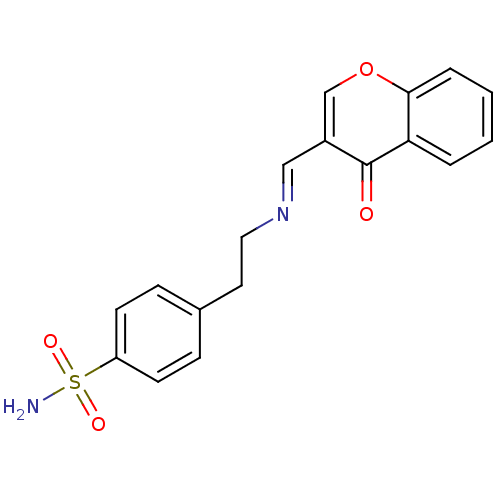
(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)ethyl)...)Show SMILES NS(=O)(=O)c1ccc(CC\N=C\c2coc3ccccc3c2=O)cc1 Show InChI InChI=1S/C18H16N2O4S/c19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21/h1-8,11-12H,9-10H2,(H2,19,22,23)/b20-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167402

(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)pyrimi...)Show SMILES NS(=O)(=O)c1ccc(Nc2ccnc(\N=C\c3coc4ccccc4c3=O)n2)cc1 Show InChI InChI=1S/C20H15N5O4S/c21-30(27,28)15-7-5-14(6-8-15)24-18-9-10-22-20(25-18)23-11-13-12-29-17-4-2-1-3-16(17)19(13)26/h1-12H,(H2,21,27,28)(H,22,24,25)/b23-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167414

(CHEMBL274255 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4Cc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:49,t:6| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,11,18H,9-10H2,1H3,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167418

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\c3nccc(Nc4ccc(cc4)S(N)(=O)=O)n3)c(=O)c2c1 Show InChI InChI=1S/C21H17N5O4S/c1-13-2-7-18-17(10-13)20(27)14(12-30-18)11-24-21-23-9-8-19(26-21)25-15-3-5-16(6-4-15)31(22,28)29/h2-12H,1H3,(H2,22,28,29)(H,23,25,26)/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167406

(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)ethyl)...)Show SMILES NS(=O)(=O)c1ccc(CC\N=C\c2coc3ccccc3c2=O)cc1 Show InChI InChI=1S/C18H16N2O4S/c19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21/h1-8,11-12H,9-10H2,(H2,19,22,23)/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167404
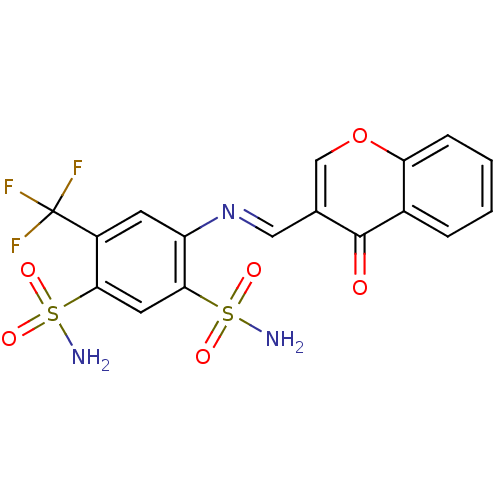
(4-((4-oxo-4H-chromen-3-yl)methyleneamino)-6-(trifl...)Show SMILES NS(=O)(=O)c1cc(c(cc1\N=C\c1coc2ccccc2c1=O)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C17H12F3N3O6S2/c18-17(19,20)11-5-12(15(31(22,27)28)6-14(11)30(21,25)26)23-7-9-8-29-13-4-2-1-3-10(13)16(9)24/h1-8H,(H2,21,25,26)(H2,22,27,28)/b23-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167412

(CHEMBL382309 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3CN(CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4CCc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:51,t:6| Show InChI InChI=1S/2C19H20N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10,12,19H,8-9,11H2,1H3,(H2,20,23,24);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167417
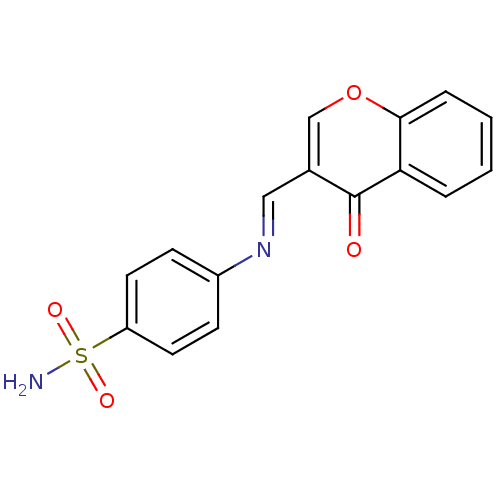
(4-((4-oxo-4H-chromen-3-yl)methyleneamino)benzenesu...)Show InChI InChI=1S/C16H12N2O4S/c17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19/h1-10H,(H2,17,20,21)/b18-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167408

(4-(((4-oxo-4H-chromen-3-yl)methyleneamino)methyl)b...)Show InChI InChI=1S/C17H14N2O4S/c18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20/h1-8,10-11H,9H2,(H2,18,21,22)/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167415

(CHEMBL371127 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4c2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:47,t:6| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-8,10,17H,9H2,1H3,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167411
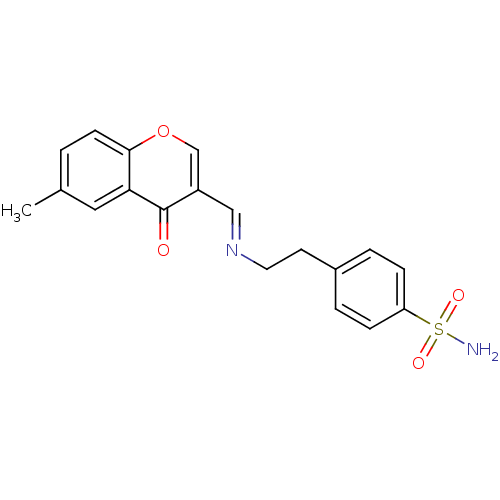
(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\CCc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C19H18N2O4S/c1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24/h2-7,10-12H,8-9H2,1H3,(H2,20,23,24)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167404

(4-((4-oxo-4H-chromen-3-yl)methyleneamino)-6-(trifl...)Show SMILES NS(=O)(=O)c1cc(c(cc1\N=C\c1coc2ccccc2c1=O)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C17H12F3N3O6S2/c18-17(19,20)11-5-12(15(31(22,27)28)6-14(11)30(21,25)26)23-7-9-8-29-13-4-2-1-3-10(13)16(9)24/h1-8H,(H2,21,25,26)(H2,22,27,28)/b23-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167423
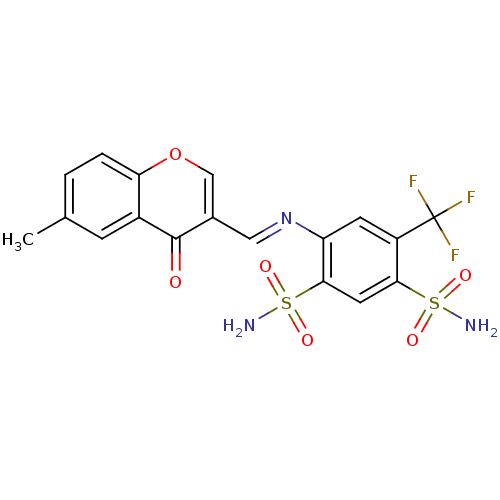
(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3cc(c(cc3S(N)(=O)=O)S(N)(=O)=O)C(F)(F)F)c(=O)c2c1 Show InChI InChI=1S/C18H14F3N3O6S2/c1-9-2-3-14-11(4-9)17(25)10(8-30-14)7-24-13-5-12(18(19,20)21)15(31(22,26)27)6-16(13)32(23,28)29/h2-8H,1H3,(H2,22,26,27)(H2,23,28,29)/b24-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167414

(CHEMBL274255 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4Cc2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:49,t:6| Show InChI InChI=1S/2C18H18N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,11,18H,9-10H2,1H3,(H2,19,22,23);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167423

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3cc(c(cc3S(N)(=O)=O)S(N)(=O)=O)C(F)(F)F)c(=O)c2c1 Show InChI InChI=1S/C18H14F3N3O6S2/c1-9-2-3-14-11(4-9)17(25)10(8-30-14)7-24-13-5-12(18(19,20)21)15(31(22,26)27)6-16(13)32(23,28)29/h2-8H,1H3,(H2,22,26,27)(H2,23,28,29)/b24-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167418

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\c3nccc(Nc4ccc(cc4)S(N)(=O)=O)n3)c(=O)c2c1 Show InChI InChI=1S/C21H17N5O4S/c1-13-2-7-18-17(10-13)20(27)14(12-30-18)11-24-21-23-9-8-19(26-21)25-15-3-5-16(6-4-15)31(22,28)29/h2-12H,1H3,(H2,22,28,29)(H,23,25,26)/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167408

(4-(((4-oxo-4H-chromen-3-yl)methyleneamino)methyl)b...)Show InChI InChI=1S/C17H14N2O4S/c18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20/h1-8,10-11H,9H2,(H2,18,21,22)/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167406

(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)ethyl)...)Show SMILES NS(=O)(=O)c1ccc(CC\N=C\c2coc3ccccc3c2=O)cc1 Show InChI InChI=1S/C18H16N2O4S/c19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21/h1-8,11-12H,9-10H2,(H2,19,22,23)/b20-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167402

(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)pyrimi...)Show SMILES NS(=O)(=O)c1ccc(Nc2ccnc(\N=C\c3coc4ccccc4c3=O)n2)cc1 Show InChI InChI=1S/C20H15N5O4S/c21-30(27,28)15-7-5-14(6-8-15)24-18-9-10-22-20(25-18)23-11-13-12-29-17-4-2-1-3-16(17)19(13)26/h1-12H,(H2,21,27,28)(H,22,24,25)/b23-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167406

(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)ethyl)...)Show SMILES NS(=O)(=O)c1ccc(CC\N=C\c2coc3ccccc3c2=O)cc1 Show InChI InChI=1S/C18H16N2O4S/c19-25(22,23)15-7-5-13(6-8-15)9-10-20-11-14-12-24-17-4-2-1-3-16(17)18(14)21/h1-8,11-12H,9-10H2,(H2,19,22,23)/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167420

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C17H14N2O4S/c1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22/h2-10H,1H3,(H2,18,21,22)/b19-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167415

(CHEMBL371127 | sulfanilamide derivative)Show SMILES Cc1ccc2OC=C3CN(c4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(CN4c2ccc(cc2)S(N)(=O)=O)=COc2ccc(C)cc12 |c:47,t:6| Show InChI InChI=1S/2C17H16N2O4S.2ClH.Zn/c2*1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22;;;/h2*2-8,10,17H,9H2,1H3,(H2,18,21,22);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167402

(4-(2-((4-oxo-4H-chromen-3-yl)methyleneamino)pyrimi...)Show SMILES NS(=O)(=O)c1ccc(Nc2ccnc(\N=C\c3coc4ccccc4c3=O)n2)cc1 Show InChI InChI=1S/C20H15N5O4S/c21-30(27,28)15-7-5-14(6-8-15)24-18-9-10-22-20(25-18)23-11-13-12-29-17-4-2-1-3-16(17)19(13)26/h1-12H,(H2,21,27,28)(H,22,24,25)/b23-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167417

(4-((4-oxo-4H-chromen-3-yl)methyleneamino)benzenesu...)Show InChI InChI=1S/C16H12N2O4S/c17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19/h1-10H,(H2,17,20,21)/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167411

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\CCc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C19H18N2O4S/c1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24/h2-7,10-12H,8-9H2,1H3,(H2,20,23,24)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167408

(4-(((4-oxo-4H-chromen-3-yl)methyleneamino)methyl)b...)Show InChI InChI=1S/C17H14N2O4S/c18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20/h1-8,10-11H,9H2,(H2,18,21,22)/b19-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167417

(4-((4-oxo-4H-chromen-3-yl)methyleneamino)benzenesu...)Show InChI InChI=1S/C16H12N2O4S/c17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19/h1-10H,(H2,17,20,21)/b18-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167418

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\c3nccc(Nc4ccc(cc4)S(N)(=O)=O)n3)c(=O)c2c1 Show InChI InChI=1S/C21H17N5O4S/c1-13-2-7-18-17(10-13)20(27)14(12-30-18)11-24-21-23-9-8-19(26-21)25-15-3-5-16(6-4-15)31(22,28)29/h2-12H,1H3,(H2,22,28,29)(H,23,25,26)/b24-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167416
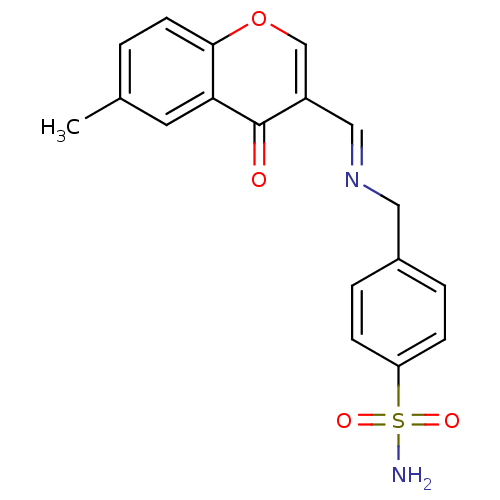
(4-(((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino...)Show SMILES Cc1ccc2occ(\C=N\Cc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C18H16N2O4S/c1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23/h2-8,10-11H,9H2,1H3,(H2,19,22,23)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167411

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\CCc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C19H18N2O4S/c1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24/h2-7,10-12H,8-9H2,1H3,(H2,20,23,24)/b21-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167416

(4-(((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino...)Show SMILES Cc1ccc2occ(\C=N\Cc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C18H16N2O4S/c1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23/h2-8,10-11H,9H2,1H3,(H2,19,22,23)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167420

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C17H14N2O4S/c1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22/h2-10H,1H3,(H2,18,21,22)/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167416

(4-(((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino...)Show SMILES Cc1ccc2occ(\C=N\Cc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C18H16N2O4S/c1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23/h2-8,10-11H,9H2,1H3,(H2,19,22,23)/b20-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167401

(CHEMBL193432 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](CCc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4CCc1ccc(cc1)S(N)(=O)=O |c:35,47,t:6,8| Show InChI InChI=1S/2C19H19N2O4S.2ClH.Zn/c2*1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24;;;/h2*2-7,10-12,19H,8-9H2,1H3,(H2,20,23,24);2*1H;/q2*-1;;;+6/p-2/b2*21-11+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167420

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C17H14N2O4S/c1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22/h2-10H,1H3,(H2,18,21,22)/b19-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167421

(CHEMBL370172 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(CN1c1ccc(cc1)S(N)(=O)=O)=COc1ccccc21 |c:46,t:13| Show InChI InChI=1S/2C16H14N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-8,10,16H,9H2,(H2,17,20,21);2*1H;/q2*-2;;;+6/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167416

(4-(((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino...)Show SMILES Cc1ccc2occ(\C=N\Cc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C18H16N2O4S/c1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23/h2-8,10-11H,9H2,1H3,(H2,19,22,23)/b20-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167411

(4-(2-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneami...)Show SMILES Cc1ccc2occ(\C=N\CCc3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C19H18N2O4S/c1-13-2-7-18-17(10-13)19(22)15(12-25-18)11-21-9-8-14-3-5-16(6-4-14)26(20,23)24/h2-7,10-12H,8-9H2,1H3,(H2,20,23,24)/b21-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167407

(CHEMBL371504 | sulfanilamide derivative )Show SMILES Cc1ccc2OC=C3C=[N+](Cc4ccc(cc4)S(N)(=O)=O)[Zn]4(Cl)(Cl)(OC3c2c1)OC1C(=COc2ccc(C)cc12)C=[N+]4Cc1ccc(cc1)S(N)(=O)=O |c:34,46,t:6,8| Show InChI InChI=1S/2C18H17N2O4S.2ClH.Zn/c2*1-12-2-7-17-16(8-12)18(21)14(11-24-17)10-20-9-13-3-5-15(6-4-13)25(19,22)23;;;/h2*2-8,10-11,18H,9H2,1H3,(H2,19,22,23);2*1H;/q2*-1;;;+6/p-2/b2*20-10+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167420

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3ccc(cc3)S(N)(=O)=O)c(=O)c2c1 Show InChI InChI=1S/C17H14N2O4S/c1-11-2-7-16-15(8-11)17(20)12(10-23-16)9-19-13-3-5-14(6-4-13)24(18,21)22/h2-10H,1H3,(H2,18,21,22)/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167423

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3cc(c(cc3S(N)(=O)=O)S(N)(=O)=O)C(F)(F)F)c(=O)c2c1 Show InChI InChI=1S/C18H14F3N3O6S2/c1-9-2-3-14-11(4-9)17(25)10(8-30-14)7-24-13-5-12(18(19,20)21)15(31(22,26)27)6-16(13)32(23,28)29/h2-8H,1H3,(H2,22,26,27)(H2,23,28,29)/b24-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167404

(4-((4-oxo-4H-chromen-3-yl)methyleneamino)-6-(trifl...)Show SMILES NS(=O)(=O)c1cc(c(cc1\N=C\c1coc2ccccc2c1=O)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C17H12F3N3O6S2/c18-17(19,20)11-5-12(15(31(22,27)28)6-14(11)30(21,25)26)23-7-9-8-29-13-4-2-1-3-10(13)16(9)24/h1-8H,(H2,21,25,26)(H2,22,27,28)/b23-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167417

(4-((4-oxo-4H-chromen-3-yl)methyleneamino)benzenesu...)Show InChI InChI=1S/C16H12N2O4S/c17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19/h1-10H,(H2,17,20,21)/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167404

(4-((4-oxo-4H-chromen-3-yl)methyleneamino)-6-(trifl...)Show SMILES NS(=O)(=O)c1cc(c(cc1\N=C\c1coc2ccccc2c1=O)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C17H12F3N3O6S2/c18-17(19,20)11-5-12(15(31(22,27)28)6-14(11)30(21,25)26)23-7-9-8-29-13-4-2-1-3-10(13)16(9)24/h1-8H,(H2,21,25,26)(H2,22,27,28)/b23-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167423

(4-((6-methyl-4-oxo-4H-chromen-3-yl)methyleneamino)...)Show SMILES Cc1ccc2occ(\C=N\c3cc(c(cc3S(N)(=O)=O)S(N)(=O)=O)C(F)(F)F)c(=O)c2c1 Show InChI InChI=1S/C18H14F3N3O6S2/c1-9-2-3-14-11(4-9)17(25)10(8-30-14)7-24-13-5-12(18(19,20)21)15(31(22,26)27)6-16(13)32(23,28)29/h2-8H,1H3,(H2,22,26,27)(H2,23,28,29)/b24-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167410
(1N-amino(imino)methyl-4-[1-(6-methyl-4-oxo-4H-3-ch...)Show SMILES Cc1ccc2occ(C=Nc3ccc(cc3)S(=O)(=O)NC(N)=N)c(=O)c2c1 |w:8.7| Show InChI InChI=1S/C18H16N4O4S/c1-11-2-7-16-15(8-11)17(23)12(10-26-16)9-21-13-3-5-14(6-4-13)27(24,25)22-18(19)20/h2-10H,1H3,(H4,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167419
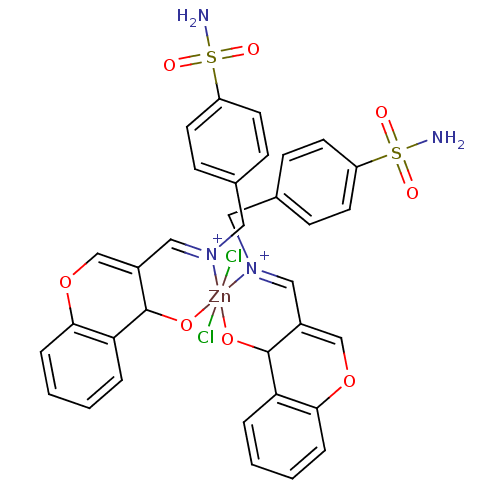
(CHEMBL369923 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(C[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2Cc2ccc(cc2)S(N)(=O)=O)cc1 |c:30,41,t:9,11| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,10-11,17H,9H2,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-10+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167403
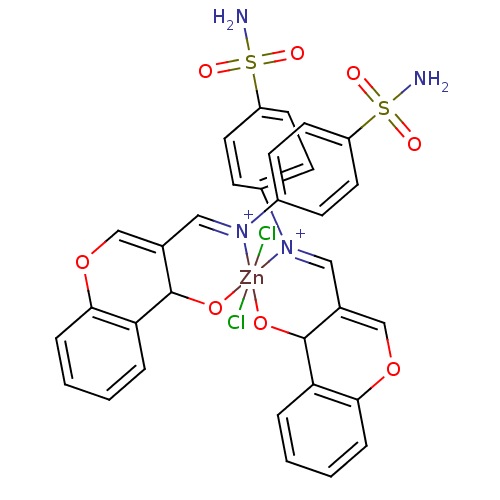
(CHEMBL369922 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(cc1)[N+]1=CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(=COc3ccccc23)C=[N+]1c1ccc(cc1)S(N)(=O)=O |c:32,43,t:11,13| Show InChI InChI=1S/2C16H13N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-10,16H,(H2,17,20,21);2*1H;/q2*-1;;;+6/p-2/b2*18-9+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167403

(CHEMBL369922 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(cc1)[N+]1=CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(=COc3ccccc23)C=[N+]1c1ccc(cc1)S(N)(=O)=O |c:32,43,t:11,13| Show InChI InChI=1S/2C16H13N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-10,16H,(H2,17,20,21);2*1H;/q2*-1;;;+6/p-2/b2*18-9+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167419

(CHEMBL369923 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(C[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2Cc2ccc(cc2)S(N)(=O)=O)cc1 |c:30,41,t:9,11| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,10-11,17H,9H2,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-10+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167403

(CHEMBL369922 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(cc1)[N+]1=CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(=COc3ccccc23)C=[N+]1c1ccc(cc1)S(N)(=O)=O |c:32,43,t:11,13| Show InChI InChI=1S/2C16H13N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-10,16H,(H2,17,20,21);2*1H;/q2*-1;;;+6/p-2/b2*18-9+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167419

(CHEMBL369923 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(C[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2Cc2ccc(cc2)S(N)(=O)=O)cc1 |c:30,41,t:9,11| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,10-11,17H,9H2,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-10+;;; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167410

(1N-amino(imino)methyl-4-[1-(6-methyl-4-oxo-4H-3-ch...)Show SMILES Cc1ccc2occ(C=Nc3ccc(cc3)S(=O)(=O)NC(N)=N)c(=O)c2c1 |w:8.7| Show InChI InChI=1S/C18H16N4O4S/c1-11-2-7-16-15(8-11)17(23)12(10-26-16)9-21-13-3-5-14(6-4-13)27(24,25)22-18(19)20/h2-10H,1H3,(H4,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50167409
(1N-amino(imino)methyl-4-[1-(4-oxo-4H-3-chromenyl)-...)Show SMILES NC(=N)NS(=O)(=O)c1ccc(cc1)\N=C\c1coc2ccccc2c1=O Show InChI InChI=1S/C17H14N4O4S/c18-17(19)21-26(23,24)13-7-5-12(6-8-13)20-9-11-10-25-15-4-2-1-3-14(15)16(11)22/h1-10H,(H4,18,19,21)/b20-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase II |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50167409

(1N-amino(imino)methyl-4-[1-(4-oxo-4H-3-chromenyl)-...)Show SMILES NC(=N)NS(=O)(=O)c1ccc(cc1)\N=C\c1coc2ccccc2c1=O Show InChI InChI=1S/C17H14N4O4S/c18-17(19)21-26(23,24)13-7-5-12(6-8-13)20-9-11-10-25-15-4-2-1-3-14(15)16(11)22/h1-10H,(H4,18,19,21)/b20-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167409

(1N-amino(imino)methyl-4-[1-(4-oxo-4H-3-chromenyl)-...)Show SMILES NC(=N)NS(=O)(=O)c1ccc(cc1)\N=C\c1coc2ccccc2c1=O Show InChI InChI=1S/C17H14N4O4S/c18-17(19)21-26(23,24)13-7-5-12(6-8-13)20-9-11-10-25-15-4-2-1-3-14(15)16(11)22/h1-10H,(H4,18,19,21)/b20-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167409

(1N-amino(imino)methyl-4-[1-(4-oxo-4H-3-chromenyl)-...)Show SMILES NC(=N)NS(=O)(=O)c1ccc(cc1)\N=C\c1coc2ccccc2c1=O Show InChI InChI=1S/C17H14N4O4S/c18-17(19)21-26(23,24)13-7-5-12(6-8-13)20-9-11-10-25-15-4-2-1-3-14(15)16(11)22/h1-10H,(H4,18,19,21)/b20-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167410

(1N-amino(imino)methyl-4-[1-(6-methyl-4-oxo-4H-3-ch...)Show SMILES Cc1ccc2occ(C=Nc3ccc(cc3)S(=O)(=O)NC(N)=N)c(=O)c2c1 |w:8.7| Show InChI InChI=1S/C18H16N4O4S/c1-11-2-7-16-15(8-11)17(23)12(10-26-16)9-21-13-3-5-14(6-4-13)27(24,25)22-18(19)20/h2-10H,1H3,(H4,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167403

(CHEMBL369922 | sulfanilamide derivative )Show SMILES NS(=O)(=O)c1ccc(cc1)[N+]1=CC2=COc3ccccc3C2O[Zn]11(Cl)(Cl)OC2C(=COc3ccccc23)C=[N+]1c1ccc(cc1)S(N)(=O)=O |c:32,43,t:11,13| Show InChI InChI=1S/2C16H13N2O4S.2ClH.Zn/c2*17-23(20,21)13-7-5-12(6-8-13)18-9-11-10-22-15-4-2-1-3-14(15)16(11)19;;;/h2*1-10,16H,(H2,17,20,21);2*1H;/q2*-1;;;+6/p-2/b2*18-9+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50167410

(1N-amino(imino)methyl-4-[1-(6-methyl-4-oxo-4H-3-ch...)Show SMILES Cc1ccc2occ(C=Nc3ccc(cc3)S(=O)(=O)NC(N)=N)c(=O)c2c1 |w:8.7| Show InChI InChI=1S/C18H16N4O4S/c1-11-2-7-16-15(8-11)17(23)12(10-26-16)9-21-13-3-5-14(6-4-13)27(24,25)22-18(19)20/h2-10H,1H3,(H4,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase IX |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50167419

(CHEMBL369923 | sulfanilamide derivative)Show SMILES NS(=O)(=O)c1ccc(C[N+]2=CC3=COc4ccccc4C3O[Zn]22(Cl)(Cl)OC3C(=COc4ccccc34)C=[N+]2Cc2ccc(cc2)S(N)(=O)=O)cc1 |c:30,41,t:9,11| Show InChI InChI=1S/2C17H15N2O4S.2ClH.Zn/c2*18-24(21,22)14-7-5-12(6-8-14)9-19-10-13-11-23-16-4-2-1-3-15(16)17(13)20;;;/h2*1-8,10-11,17H,9H2,(H2,18,21,22);2*1H;/q2*-1;;;+6/p-2/b2*19-10+;;; | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase XII |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ospedale San Lazzaro
Curated by ChEMBL
| Assay Description
Inhibition constant against human carbonic anhydrase I |
Bioorg Med Chem Lett 15: 3096-101 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.055
BindingDB Entry DOI: 10.7270/Q27M07FQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data