 Found 131 hits with Last Name = 'wild' and Initial = 'h'
Found 131 hits with Last Name = 'wild' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095409
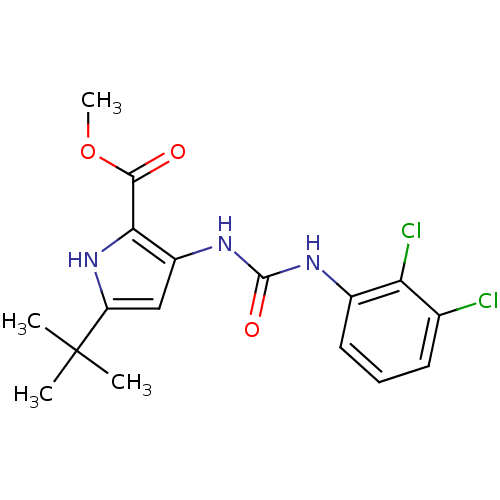
(5-tert-Butyl-3-[3-(2,3-dichloro-phenyl)-ureido]-1H...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C17H19Cl2N3O3/c1-17(2,3)12-8-11(14(22-12)15(23)25-4)21-16(24)20-10-7-5-6-9(18)13(10)19/h5-8,22H,1-4H3,(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50358043
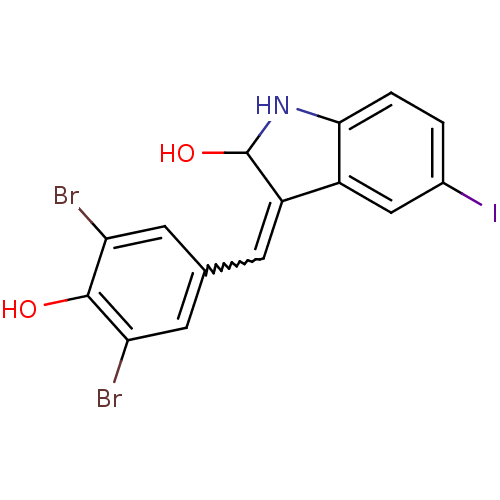
(CHEMBL1794051 | GW-5074)Show InChI InChI=1S/C15H10Br2INO2/c16-11-4-7(5-12(17)14(11)20)3-10-9-6-8(18)1-2-13(9)19-15(10)21/h1-6,15,19-21H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against raf kinase. |
Bioorg Med Chem Lett 11: 2775-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NS0VD5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095390
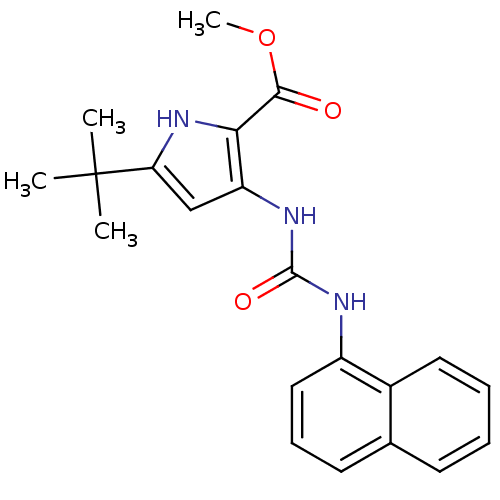
(5-tert-Butyl-3-(3-naphthalen-1-yl-ureido)-1H-pyrro...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1cccc2ccccc12)C(C)(C)C Show InChI InChI=1S/C21H23N3O3/c1-21(2,3)17-12-16(18(24-17)19(25)27-4)23-20(26)22-15-11-7-9-13-8-5-6-10-14(13)15/h5-12,24H,1-4H3,(H2,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
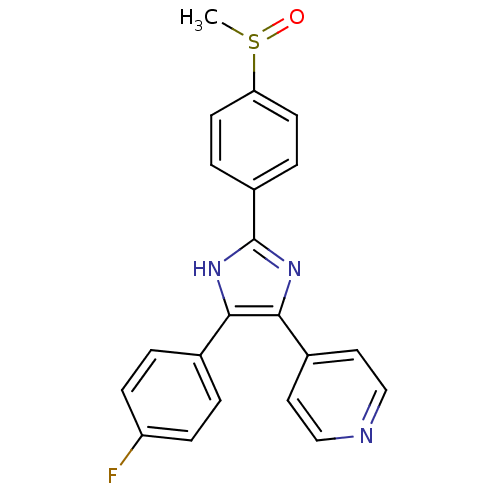
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095392
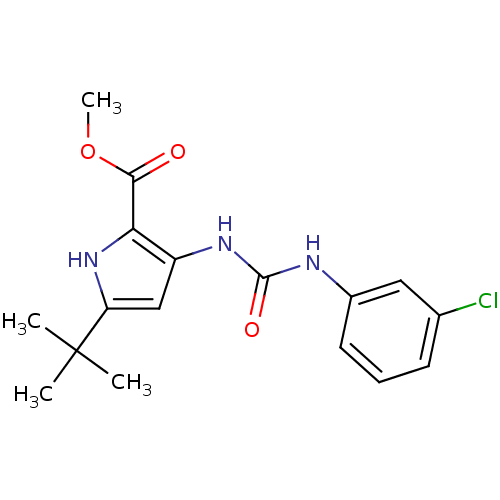
(5-tert-Butyl-3-[3-(3-chloro-phenyl)-ureido]-1H-pyr...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1cccc(Cl)c1)C(C)(C)C Show InChI InChI=1S/C17H20ClN3O3/c1-17(2,3)13-9-12(14(21-13)15(22)24-4)20-16(23)19-11-7-5-6-10(18)8-11/h5-9,21H,1-4H3,(H2,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095415
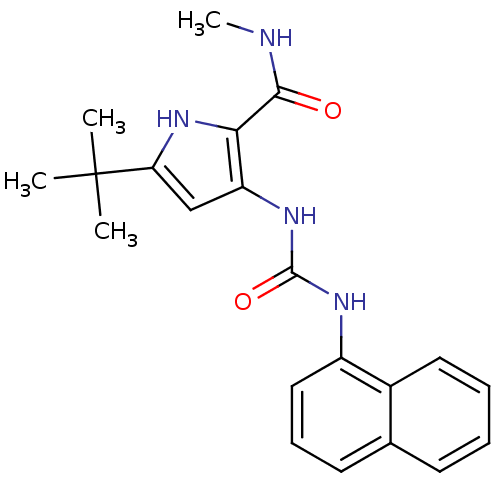
(5-tert-Butyl-3-(3-naphthalen-1-yl-ureido)-1H-pyrro...)Show SMILES CNC(=O)c1[nH]c(cc1NC(=O)Nc1cccc2ccccc12)C(C)(C)C Show InChI InChI=1S/C21H24N4O2/c1-21(2,3)17-12-16(18(25-17)19(26)22-4)24-20(27)23-15-11-7-9-13-8-5-6-10-14(13)15/h5-12,25H,1-4H3,(H,22,26)(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095402
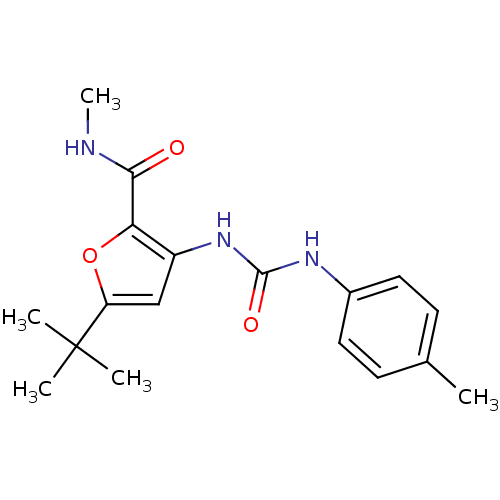
(5-tert-Butyl-3-(3-p-tolyl-ureido)-furan-2-carboxyl...)Show InChI InChI=1S/C18H23N3O3/c1-11-6-8-12(9-7-11)20-17(23)21-13-10-14(18(2,3)4)24-15(13)16(22)19-5/h6-10H,1-5H3,(H,19,22)(H2,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095429
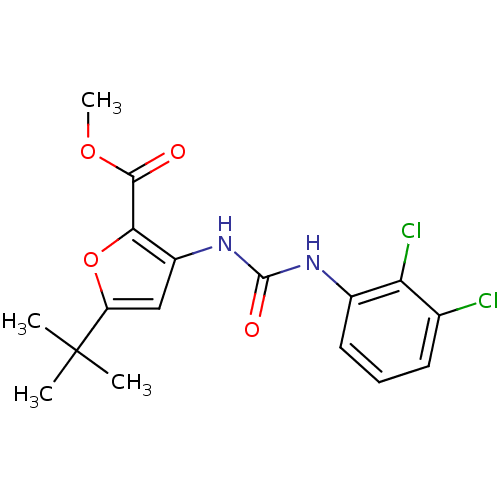
(5-tert-Butyl-3-[3-(2,3-dichloro-phenyl)-ureido]-fu...)Show SMILES COC(=O)c1oc(cc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C17H18Cl2N2O4/c1-17(2,3)12-8-11(14(25-12)15(22)24-4)21-16(23)20-10-7-5-6-9(18)13(10)19/h5-8H,1-4H3,(H2,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095428
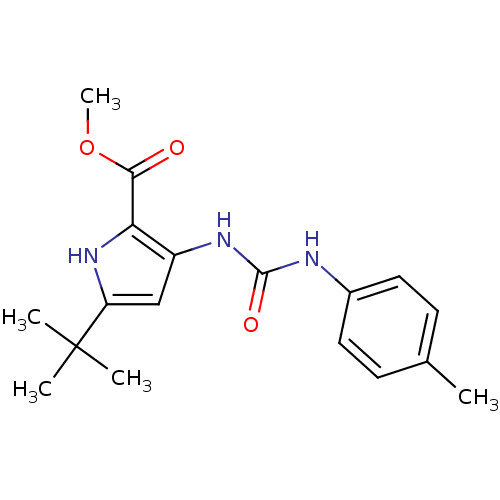
(5-tert-Butyl-3-(3-p-tolyl-ureido)-1H-pyrrole-2-car...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1ccc(C)cc1)C(C)(C)C Show InChI InChI=1S/C18H23N3O3/c1-11-6-8-12(9-7-11)19-17(23)20-13-10-14(18(2,3)4)21-15(13)16(22)24-5/h6-10,21H,1-5H3,(H2,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095403
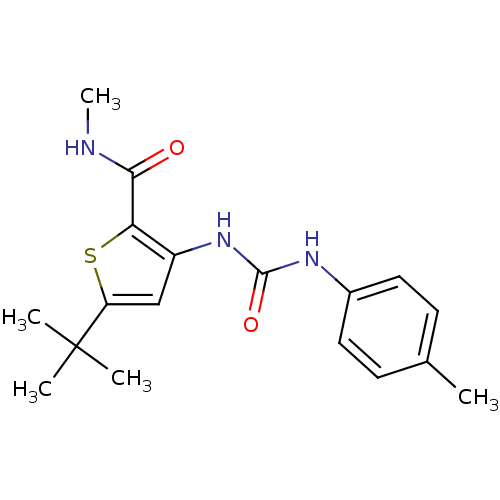
(1-(5-tert-butyl-2-(methylcarbamoyl)thiophen-3-yl)-...)Show InChI InChI=1S/C18H23N3O2S/c1-11-6-8-12(9-7-11)20-17(23)21-13-10-14(18(2,3)4)24-15(13)16(22)19-5/h6-10H,1-5H3,(H,19,22)(H2,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095398
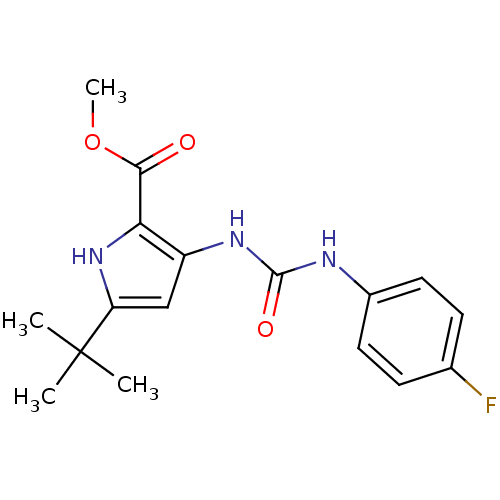
(5-tert-Butyl-3-[3-(4-fluoro-phenyl)-ureido]-1H-pyr...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1ccc(F)cc1)C(C)(C)C Show InChI InChI=1S/C17H20FN3O3/c1-17(2,3)13-9-12(14(21-13)15(22)24-4)20-16(23)19-11-7-5-10(18)6-8-11/h5-9,21H,1-4H3,(H2,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095407
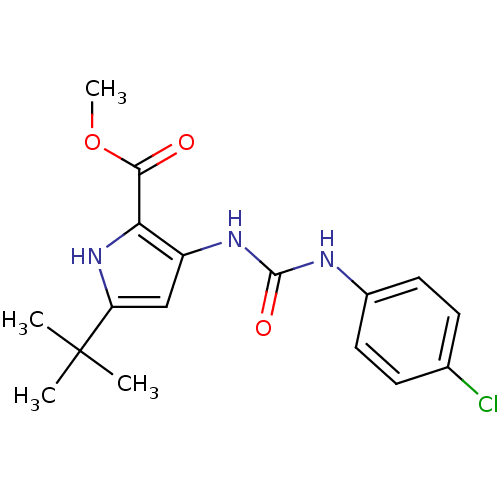
(5-tert-Butyl-3-[3-(4-chloro-phenyl)-ureido]-1H-pyr...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C17H20ClN3O3/c1-17(2,3)13-9-12(14(21-13)15(22)24-4)20-16(23)19-11-7-5-10(18)6-8-11/h5-9,21H,1-4H3,(H2,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095397
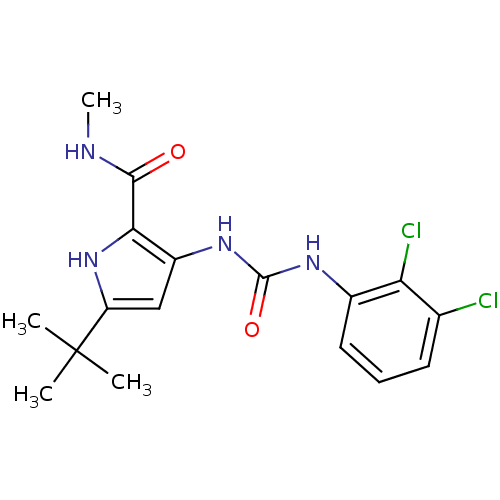
(5-tert-Butyl-3-[3-(2,3-dichloro-phenyl)-ureido]-1H...)Show SMILES CNC(=O)c1[nH]c(cc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C17H20Cl2N4O2/c1-17(2,3)12-8-11(14(23-12)15(24)20-4)22-16(25)21-10-7-5-6-9(18)13(10)19/h5-8,23H,1-4H3,(H,20,24)(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095426
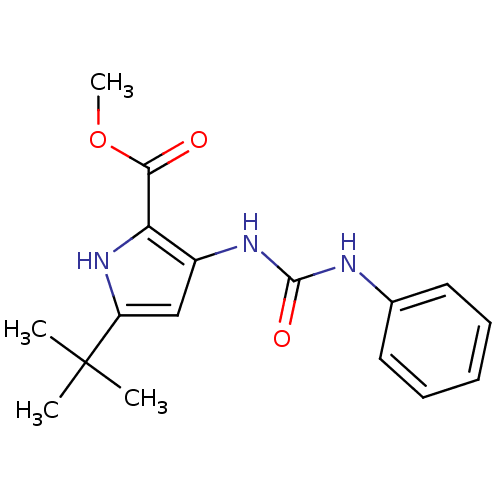
(5-tert-Butyl-3-(3-phenyl-ureido)-1H-pyrrole-2-carb...)Show InChI InChI=1S/C17H21N3O3/c1-17(2,3)13-10-12(14(20-13)15(21)23-4)19-16(22)18-11-8-6-5-7-9-11/h5-10,20H,1-4H3,(H2,18,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118338

(US8653111, 62)Show SMILES CC(C)(C)OC(=O)c1ccc(nc1)-n1[nH]cc(-n2ccnn2)c1=O Show InChI InChI=1S/C15H16N6O3/c1-15(2,3)24-14(23)10-4-5-12(16-8-10)21-13(22)11(9-18-21)20-7-6-17-19-20/h4-9,18H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095401
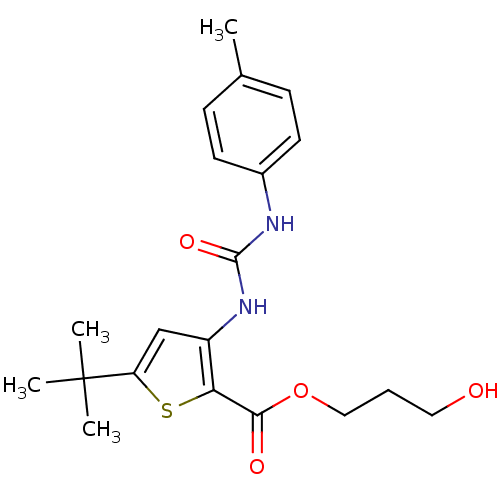
(5-tert-Butyl-3-(3-p-tolyl-ureido)-thiophene-2-carb...)Show SMILES Cc1ccc(NC(=O)Nc2cc(sc2C(=O)OCCCO)C(C)(C)C)cc1 Show InChI InChI=1S/C20H26N2O4S/c1-13-6-8-14(9-7-13)21-19(25)22-15-12-16(20(2,3)4)27-17(15)18(24)26-11-5-10-23/h6-9,12,23H,5,10-11H2,1-4H3,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095412
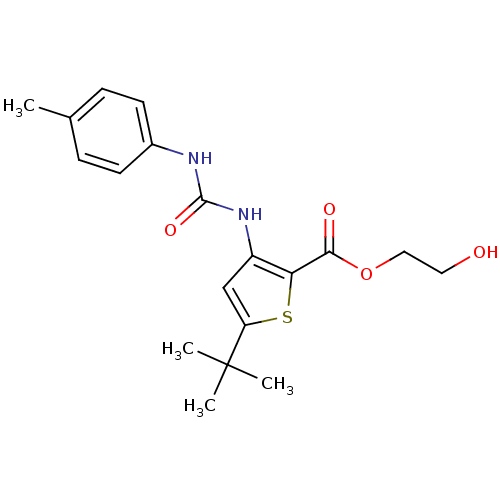
(5-tert-Butyl-3-(3-p-tolyl-ureido)-thiophene-2-carb...)Show SMILES Cc1ccc(NC(=O)Nc2cc(sc2C(=O)OCCO)C(C)(C)C)cc1 Show InChI InChI=1S/C19H24N2O4S/c1-12-5-7-13(8-6-12)20-18(24)21-14-11-15(19(2,3)4)26-16(14)17(23)25-10-9-22/h5-8,11,22H,9-10H2,1-4H3,(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095424
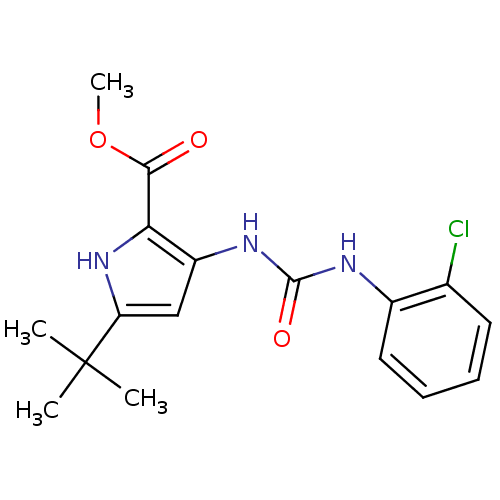
(5-tert-Butyl-3-[3-(2-chloro-phenyl)-ureido]-1H-pyr...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1ccccc1Cl)C(C)(C)C Show InChI InChI=1S/C17H20ClN3O3/c1-17(2,3)13-9-12(14(21-13)15(22)24-4)20-16(23)19-11-8-6-5-7-10(11)18/h5-9,21H,1-4H3,(H2,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095408
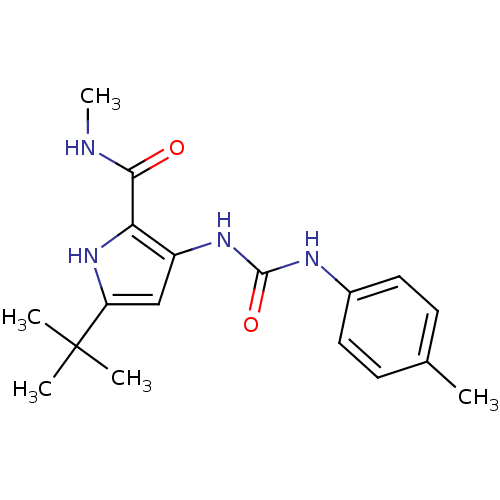
(5-tert-Butyl-3-(3-p-tolyl-ureido)-1H-pyrrole-2-car...)Show SMILES CNC(=O)c1[nH]c(cc1NC(=O)Nc1ccc(C)cc1)C(C)(C)C Show InChI InChI=1S/C18H24N4O2/c1-11-6-8-12(9-7-11)20-17(24)21-13-10-14(18(2,3)4)22-15(13)16(23)19-5/h6-10,22H,1-5H3,(H,19,23)(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095396
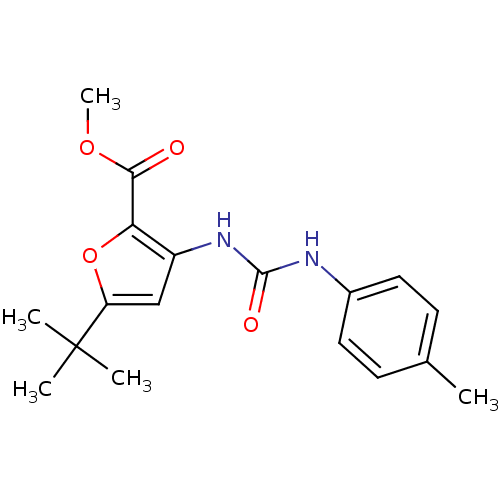
(5-tert-Butyl-3-(3-p-tolyl-ureido)-furan-2-carboxyl...)Show InChI InChI=1S/C18H22N2O4/c1-11-6-8-12(9-7-11)19-17(22)20-13-10-14(18(2,3)4)24-15(13)16(21)23-5/h6-10H,1-5H3,(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095391
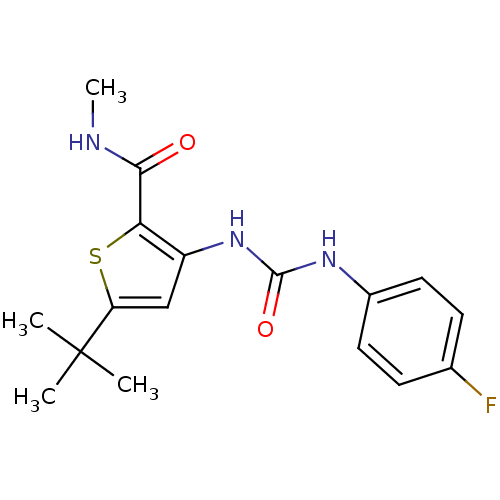
(5-tert-Butyl-3-[3-(4-fluoro-phenyl)-ureido]-thioph...)Show InChI InChI=1S/C17H20FN3O2S/c1-17(2,3)13-9-12(14(24-13)15(22)19-4)21-16(23)20-11-7-5-10(18)6-8-11/h5-9H,1-4H3,(H,19,22)(H2,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095422
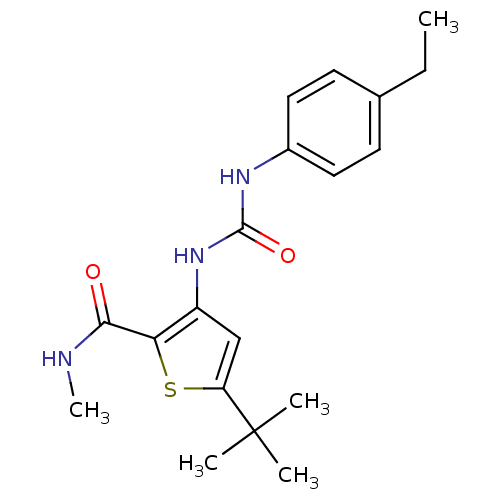
(5-tert-Butyl-3-[3-(4-ethyl-phenyl)-ureido]-thiophe...)Show SMILES CCc1ccc(NC(=O)Nc2cc(sc2C(=O)NC)C(C)(C)C)cc1 Show InChI InChI=1S/C19H25N3O2S/c1-6-12-7-9-13(10-8-12)21-18(24)22-14-11-15(19(2,3)4)25-16(14)17(23)20-5/h7-11H,6H2,1-5H3,(H,20,23)(H2,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095406
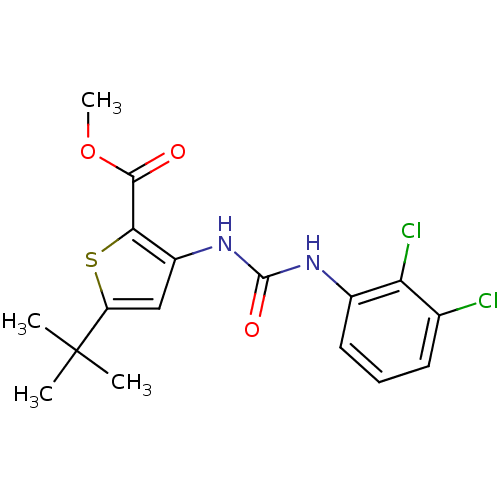
(5-tert-Butyl-3-[3-(2,3-dichloro-phenyl)-ureido]-th...)Show SMILES COC(=O)c1sc(cc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C17H18Cl2N2O3S/c1-17(2,3)12-8-11(14(25-12)15(22)24-4)21-16(23)20-10-7-5-6-9(18)13(10)19/h5-8H,1-4H3,(H2,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Transmembrane prolyl 4-hydroxylase
(Homo sapiens (Human)) | BDBM3527

(US8524699, 18)Show InChI InChI=1S/C16H16N6O2/c23-16-13(12-2-1-3-17-9-12)10-20-22(16)15-8-14(18-11-19-15)21-4-6-24-7-5-21/h1-3,8-11,20H,4-7H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. |
US Patent US8524699 (2013)
BindingDB Entry DOI: 10.7270/Q2TB15J4 |
More data for this
Ligand-Target Pair | |
von Hippel-Lindau disease tumor suppressor
(Homo sapiens (Human)) | BDBM171824

(US9085572, 34)Show InChI InChI=1S/C15H11N5O/c1-10-4-5-17-14(6-10)20-15(21)13(9-19-20)11-2-3-12(7-16)18-8-11/h2-6,8-9,19H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US9085572 (2015)
BindingDB Entry DOI: 10.7270/Q2H70DMQ |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118341
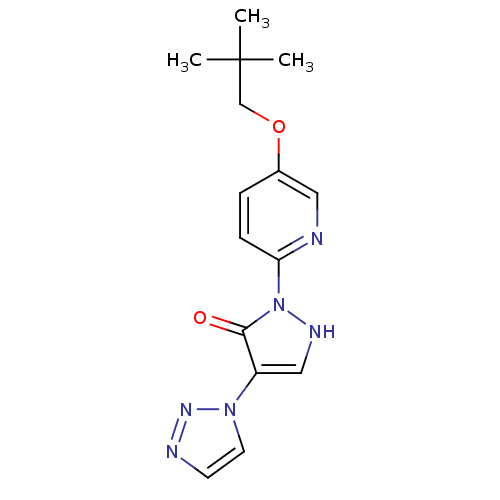
(US8653111, 85)Show SMILES CC(C)(C)COc1ccc(nc1)-n1[nH]cc(-n2ccnn2)c1=O Show InChI InChI=1S/C15H18N6O2/c1-15(2,3)10-23-11-4-5-13(16-8-11)21-14(22)12(9-18-21)20-7-6-17-19-20/h4-9,18H,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095418
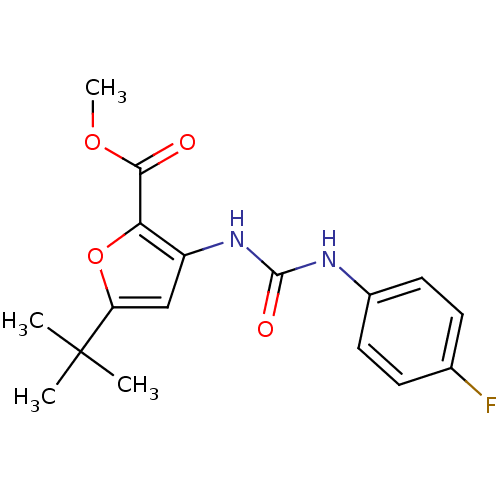
(5-tert-Butyl-3-[3-(4-fluoro-phenyl)-ureido]-furan-...)Show InChI InChI=1S/C17H19FN2O4/c1-17(2,3)13-9-12(14(24-13)15(21)23-4)20-16(22)19-11-7-5-10(18)6-8-11/h5-9H,1-4H3,(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095420
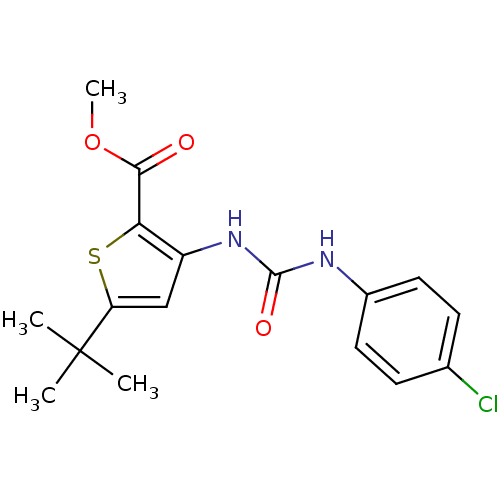
(5-tert-Butyl-3-[3-(4-chloro-phenyl)-ureido]-thioph...)Show SMILES COC(=O)c1sc(cc1NC(=O)Nc1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C17H19ClN2O3S/c1-17(2,3)13-9-12(14(24-13)15(21)23-4)20-16(22)19-11-7-5-10(18)6-8-11/h5-9H,1-4H3,(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095399
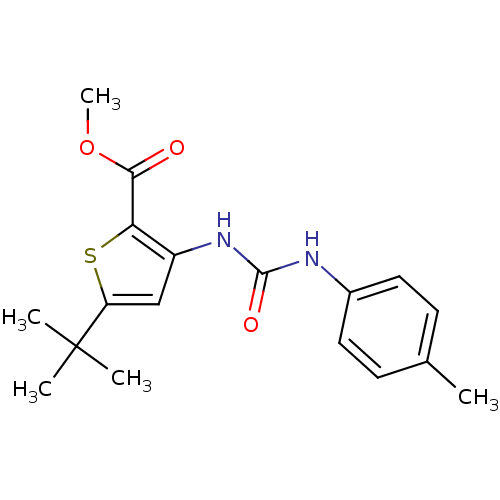
(5-tert-Butyl-3-(3-p-tolyl-ureido)-thiophene-2-carb...)Show InChI InChI=1S/C18H22N2O3S/c1-11-6-8-12(9-7-11)19-17(22)20-13-10-14(18(2,3)4)24-15(13)16(21)23-5/h6-10H,1-5H3,(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of p38 alpha2 kinase |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095400
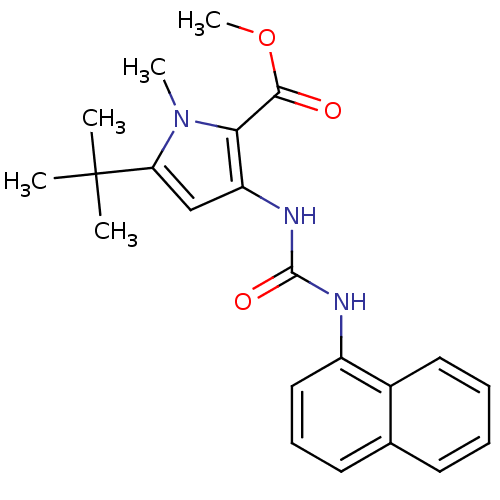
(5-tert-Butyl-1-methyl-3-(3-naphthalen-1-yl-ureido)...)Show SMILES COC(=O)c1c(NC(=O)Nc2cccc3ccccc23)cc(n1C)C(C)(C)C Show InChI InChI=1S/C22H25N3O3/c1-22(2,3)18-13-17(19(25(18)4)20(26)28-5)24-21(27)23-16-12-8-10-14-9-6-7-11-15(14)16/h6-13H,1-5H3,(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Transmembrane prolyl 4-hydroxylase
(Homo sapiens (Human)) | BDBM109268
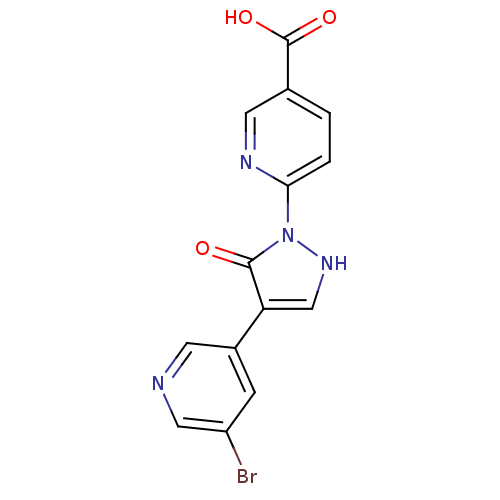
(US8609698, 10)Show SMILES OC(=O)c1ccc(nc1)-n1[nH]cc(-c2cncc(Br)c2)c1=O Show InChI InChI=1S/C14H9BrN4O3/c15-10-3-9(4-16-6-10)11-7-18-19(13(11)20)12-2-1-8(5-17-12)14(21)22/h1-7,18H,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Inhibition of the Activity of HIF Prolyl Hydroxylase is carried out as described [Oehme F., Jonghaus W., Narouz-Ott L., Huetter J., Flamme I., Anal. ... |
US Patent US8609698 (2013)
BindingDB Entry DOI: 10.7270/Q2G44NZ8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095421
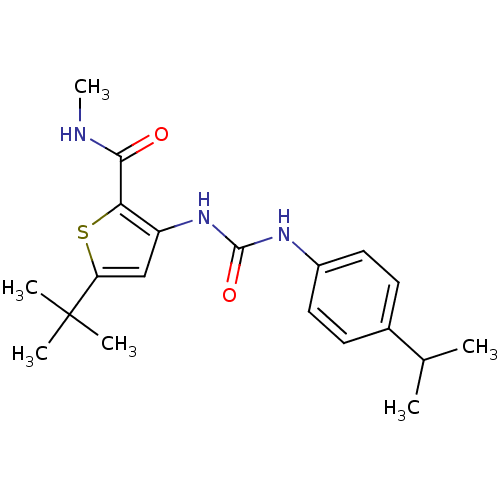
(5-tert-Butyl-3-[3-(4-isopropyl-phenyl)-ureido]-thi...)Show SMILES CNC(=O)c1sc(cc1NC(=O)Nc1ccc(cc1)C(C)C)C(C)(C)C Show InChI InChI=1S/C20H27N3O2S/c1-12(2)13-7-9-14(10-8-13)22-19(25)23-15-11-16(20(3,4)5)26-17(15)18(24)21-6/h7-12H,1-6H3,(H,21,24)(H2,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095423
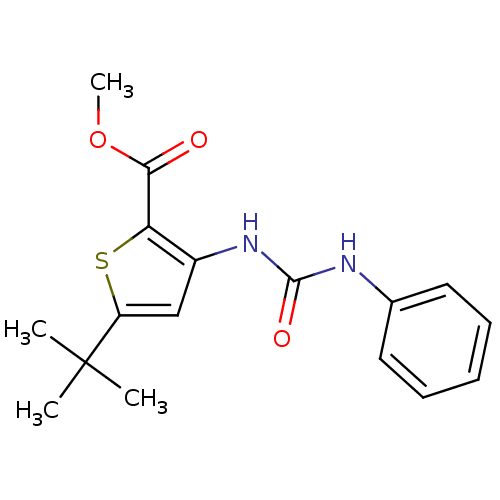
(5-tert-Butyl-3-(3-phenyl-ureido)-thiophene-2-carbo...)Show InChI InChI=1S/C17H20N2O3S/c1-17(2,3)13-10-12(14(23-13)15(20)22-4)19-16(21)18-11-8-6-5-7-9-11/h5-10H,1-4H3,(H2,18,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50095423

(5-tert-Butyl-3-(3-phenyl-ureido)-thiophene-2-carbo...)Show InChI InChI=1S/C17H20N2O3S/c1-17(2,3)13-10-12(14(23-13)15(20)22-4)19-16(21)18-11-8-6-5-7-9-11/h5-10H,1-4H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 2775-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NS0VD5 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118328

(US8653111, 7)Show InChI InChI=1S/C11H10N6O/c1-8-2-3-12-10(6-8)17-11(18)9(7-14-17)16-5-4-13-15-16/h2-7,14H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118344

(US8653111, 103)Show InChI InChI=1S/C11H11N7OS/c1-2-20-10-5-9(12-7-13-10)18-11(19)8(6-15-18)17-4-3-14-16-17/h3-7,15H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118346
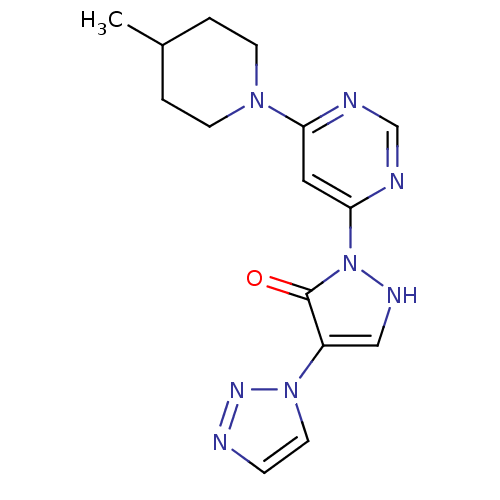
(US8653111, 129)Show SMILES CC1CCN(CC1)c1cc(ncn1)-n1[nH]cc(-n2ccnn2)c1=O Show InChI InChI=1S/C15H18N8O/c1-11-2-5-21(6-3-11)13-8-14(17-10-16-13)23-15(24)12(9-19-23)22-7-4-18-20-22/h4,7-11,19H,2-3,5-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50105619
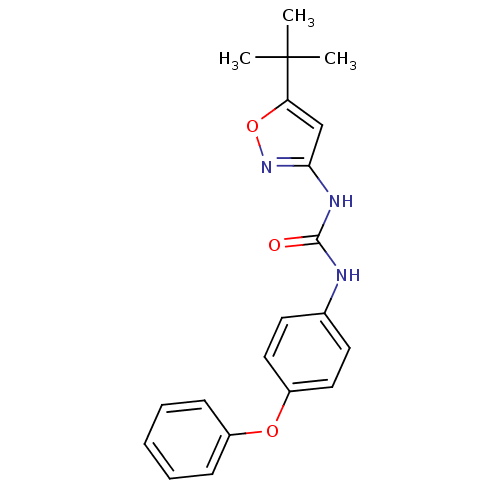
(1-(5-tert-Butyl-isoxazol-3-yl)-3-(4-phenoxy-phenyl...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ccccc3)cc2)no1 Show InChI InChI=1S/C20H21N3O3/c1-20(2,3)17-13-18(23-26-17)22-19(24)21-14-9-11-16(12-10-14)25-15-7-5-4-6-8-15/h4-13H,1-3H3,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli derived Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 11: 2775-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NS0VD5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095427
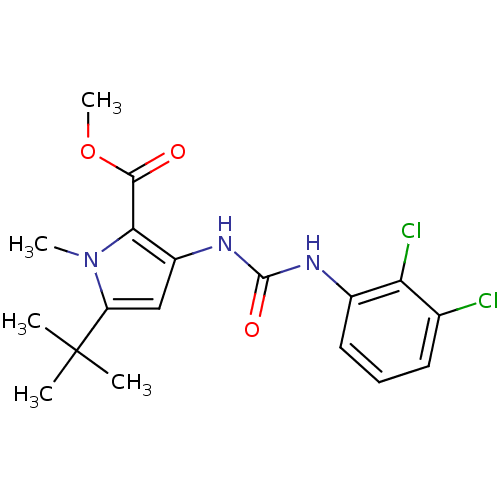
(5-tert-Butyl-3-[3-(2,3-dichloro-phenyl)-ureido]-1-...)Show SMILES COC(=O)c1c(NC(=O)Nc2cccc(Cl)c2Cl)cc(n1C)C(C)(C)C Show InChI InChI=1S/C18H21Cl2N3O3/c1-18(2,3)13-9-12(15(23(13)4)16(24)26-5)22-17(25)21-11-8-6-7-10(19)14(11)20/h6-9H,1-5H3,(H2,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095430
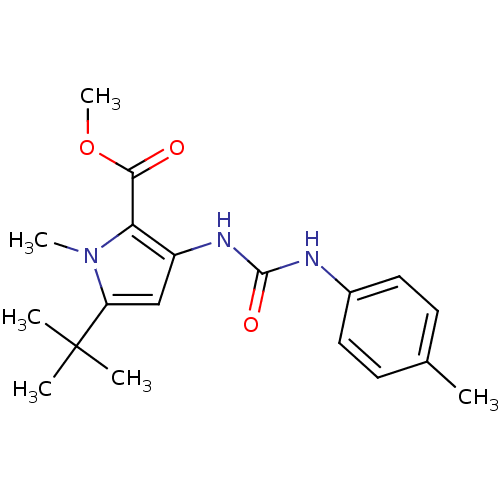
(5-tert-Butyl-1-methyl-3-(3-p-tolyl-ureido)-1H-pyrr...)Show SMILES COC(=O)c1c(NC(=O)Nc2ccc(C)cc2)cc(n1C)C(C)(C)C Show InChI InChI=1S/C19H25N3O3/c1-12-7-9-13(10-8-12)20-18(24)21-14-11-15(19(2,3)4)22(5)16(14)17(23)25-6/h7-11H,1-6H3,(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095399

(5-tert-Butyl-3-(3-p-tolyl-ureido)-thiophene-2-carb...)Show InChI InChI=1S/C18H22N2O3S/c1-11-6-8-12(9-7-11)19-17(22)20-13-10-14(18(2,3)4)24-15(13)16(21)23-5/h6-10H,1-5H3,(H2,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
von Hippel-Lindau disease tumor suppressor
(Homo sapiens (Human)) | BDBM171821
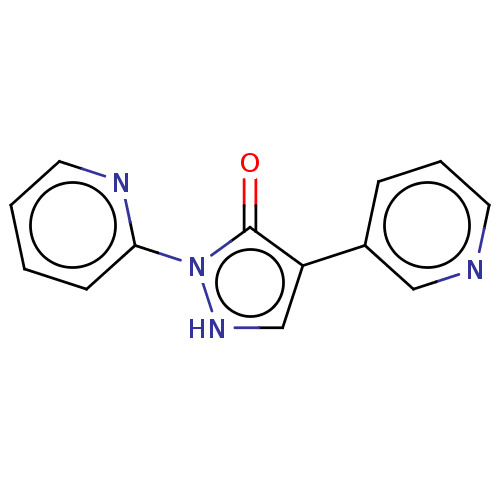
(US9085572, 6)Show InChI InChI=1S/C13H10N4O/c18-13-11(10-4-3-6-14-8-10)9-16-17(13)12-5-1-2-7-15-12/h1-9,16H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US9085572 (2015)
BindingDB Entry DOI: 10.7270/Q2H70DMQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095405
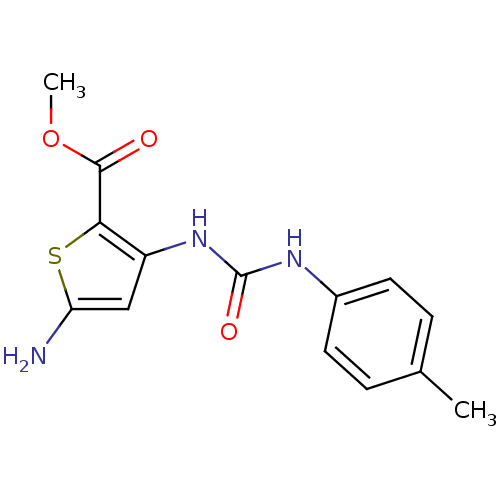
(5-Amino-3-(3-p-tolyl-ureido)-thiophene-2-carboxyli...)Show InChI InChI=1S/C14H15N3O3S/c1-8-3-5-9(6-4-8)16-14(19)17-10-7-11(15)21-12(10)13(18)20-2/h3-7H,15H2,1-2H3,(H2,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095419
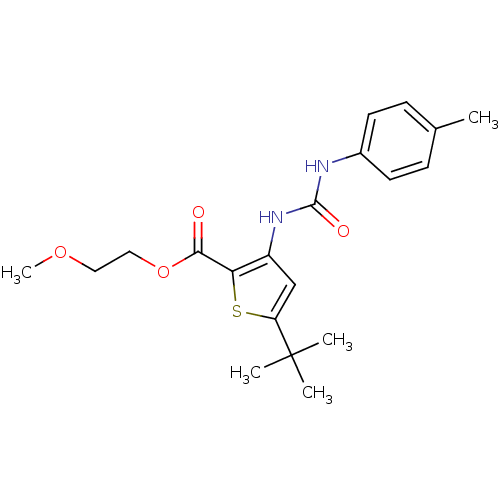
(5-tert-Butyl-3-(3-p-tolyl-ureido)-thiophene-2-carb...)Show SMILES COCCOC(=O)c1sc(cc1NC(=O)Nc1ccc(C)cc1)C(C)(C)C Show InChI InChI=1S/C20H26N2O4S/c1-13-6-8-14(9-7-13)21-19(24)22-15-12-16(20(2,3)4)27-17(15)18(23)26-11-10-25-5/h6-9,12H,10-11H2,1-5H3,(H2,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 464 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Transmembrane prolyl 4-hydroxylase
(Homo sapiens (Human)) | BDBM3543
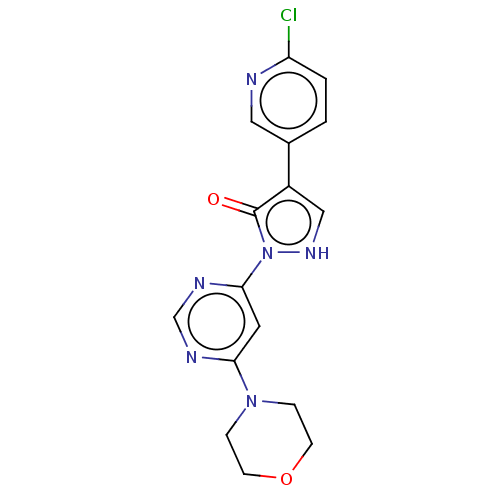
(US8524699, 16)Show SMILES Clc1ccc(cn1)-c1c[nH]n(-c2cc(ncn2)N2CCOCC2)c1=O Show InChI InChI=1S/C16H15ClN6O2/c17-13-2-1-11(8-18-13)12-9-21-23(16(12)24)15-7-14(19-10-20-15)22-3-5-25-6-4-22/h1-2,7-10,21H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. |
US Patent US8524699 (2013)
BindingDB Entry DOI: 10.7270/Q2TB15J4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095404
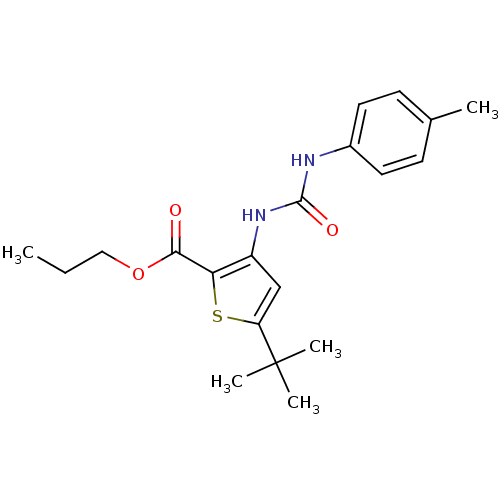
(5-tert-Butyl-3-(3-p-tolyl-ureido)-thiophene-2-carb...)Show SMILES CCCOC(=O)c1sc(cc1NC(=O)Nc1ccc(C)cc1)C(C)(C)C Show InChI InChI=1S/C20H26N2O3S/c1-6-11-25-18(23)17-15(12-16(26-17)20(3,4)5)22-19(24)21-14-9-7-13(2)8-10-14/h7-10,12H,6,11H2,1-5H3,(H2,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 alpha 2 |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118339

(US8653111, 72)Show InChI InChI=1S/C13H14N8O2/c22-13-10(20-2-1-16-18-20)8-17-21(13)12-7-11(14-9-15-12)19-3-5-23-6-4-19/h1-2,7-9,17H,3-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118329

(US8653111, 10)Show InChI InChI=1S/C15H17N7O/c23-15-12(21-7-4-16-11-21)9-19-22(15)14-8-13(17-10-18-14)20-5-2-1-3-6-20/h4,7-11,19H,1-3,5-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50105619

(1-(5-tert-Butyl-isoxazol-3-yl)-3-(4-phenoxy-phenyl...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Oc3ccccc3)cc2)no1 Show InChI InChI=1S/C20H21N3O3/c1-20(2,3)17-13-18(23-26-17)22-19(24)21-14-9-11-16(12-10-14)25-15-7-5-4-6-8-15/h4-13H,1-3H3,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against raf kinase. |
Bioorg Med Chem Lett 11: 2775-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NS0VD5 |
More data for this
Ligand-Target Pair | |
Prolyl hydroxylase EGLN2
(Homo sapiens (Human)) | BDBM118331

(US8653111, 17)Show InChI InChI=1S/C12H11N7O/c20-12-10(18-4-3-15-17-18)6-16-19(12)11-5-9(8-1-2-8)13-7-14-11/h3-8,16H,1-2H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... |
US Patent US8653111 (2014)
BindingDB Entry DOI: 10.7270/Q2GH9GMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data