

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50118719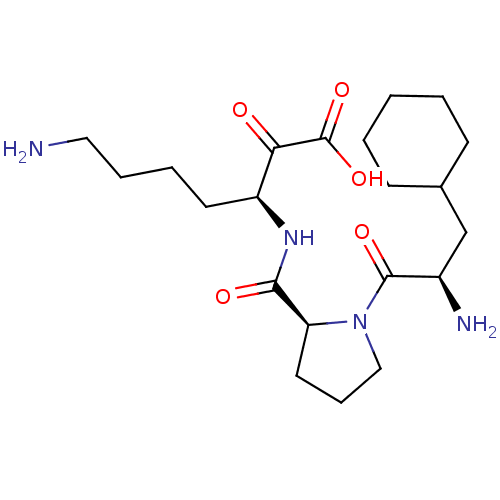 (7-Amino-3-{[1-(2-amino-3-cyclohexyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118730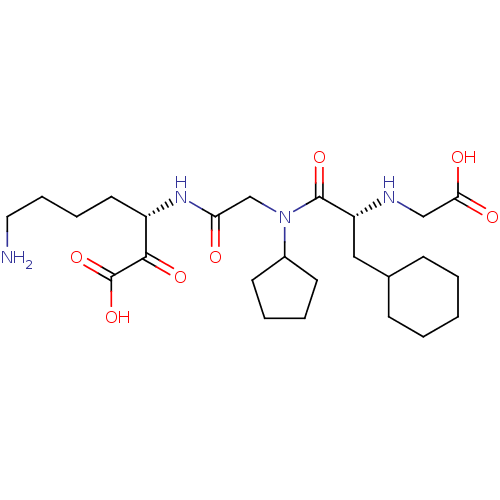 (7-Amino-3-(2-{[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118739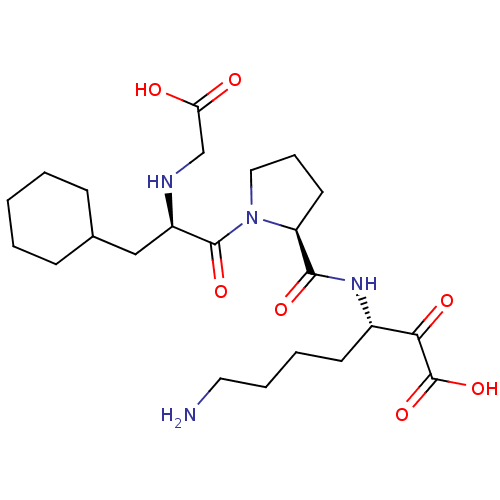 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118728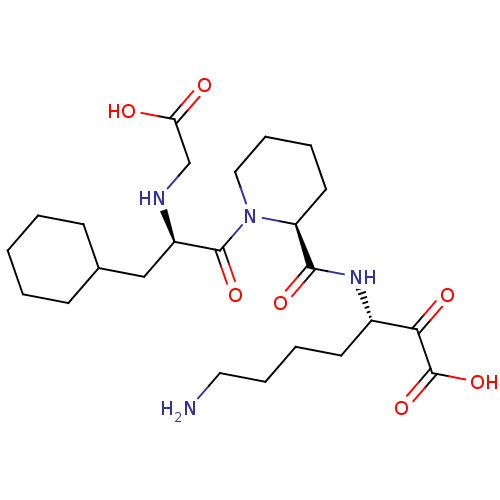 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118732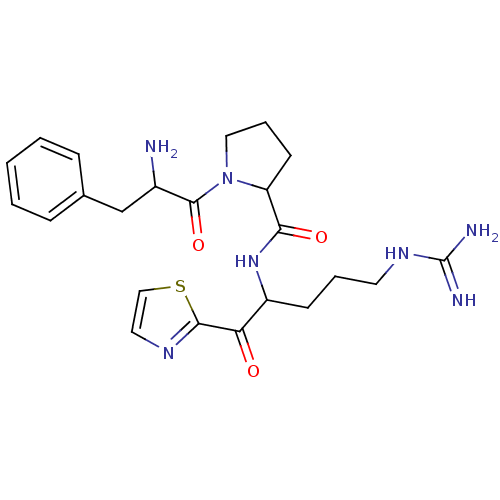 (1-(2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118729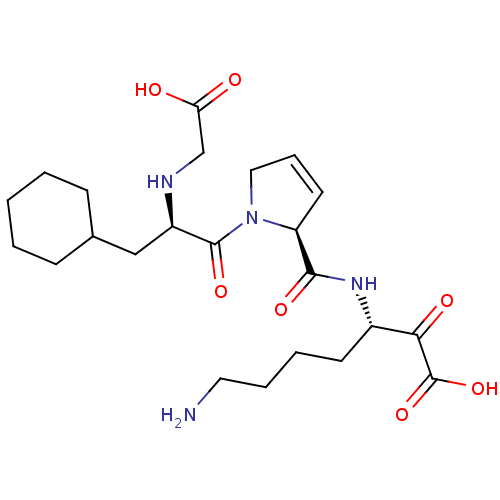 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118731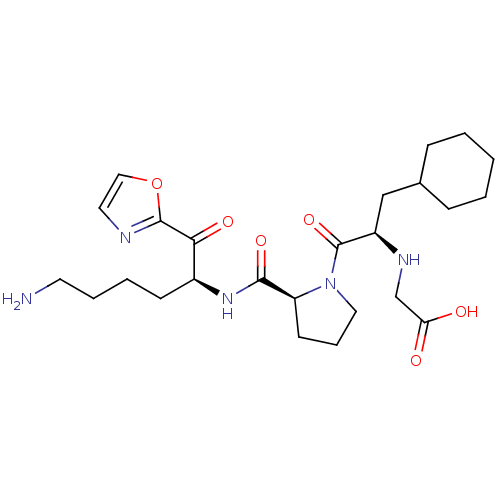 ((2-{2-[5-Amino-1-(oxazole-2-carbonyl)-pentylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118735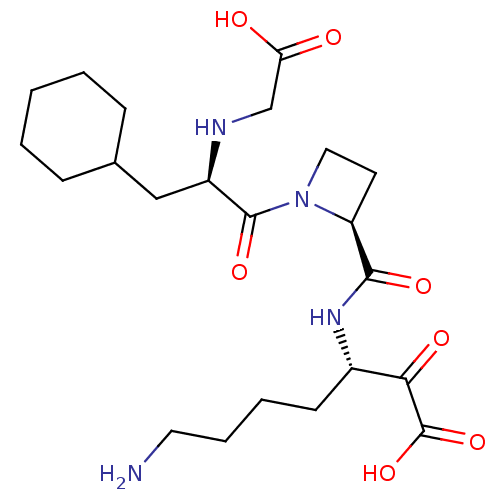 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118738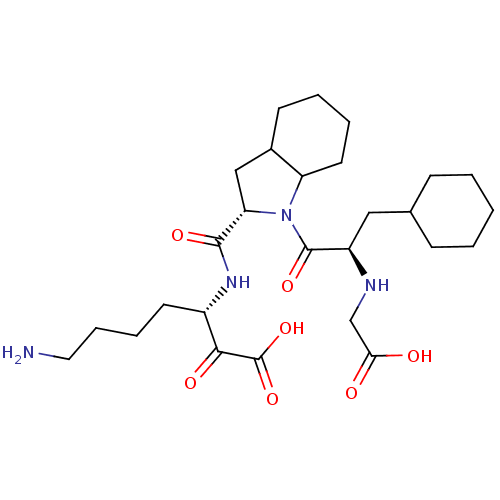 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118718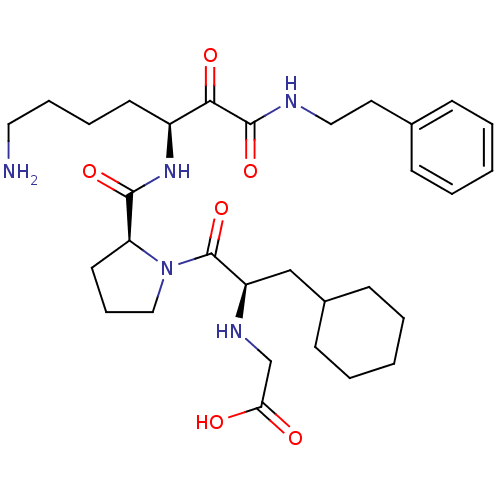 (CHEMBL343804 | {2-[2-(5-Amino-1-phenethylaminooxal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118723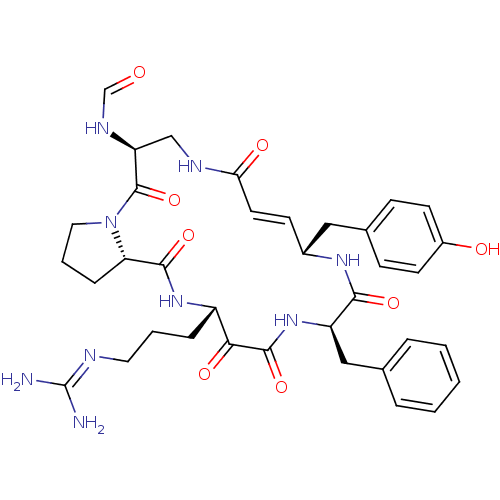 (CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118727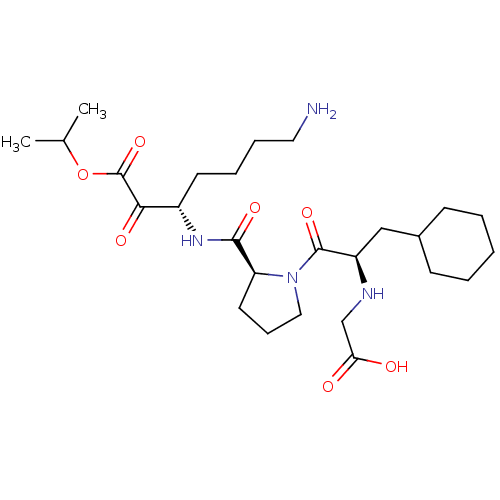 (2-((R)-1-((S)-2-(((S)-7-amino-1-isopropoxy-1,2-dio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118720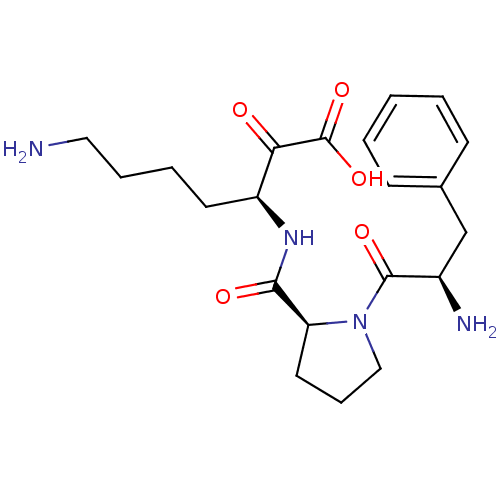 (7-Amino-3-{[1-(2-amino-3-phenyl-propionyl)-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118717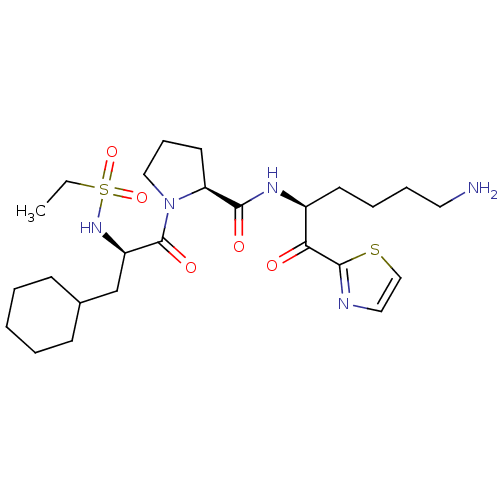 ((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM29388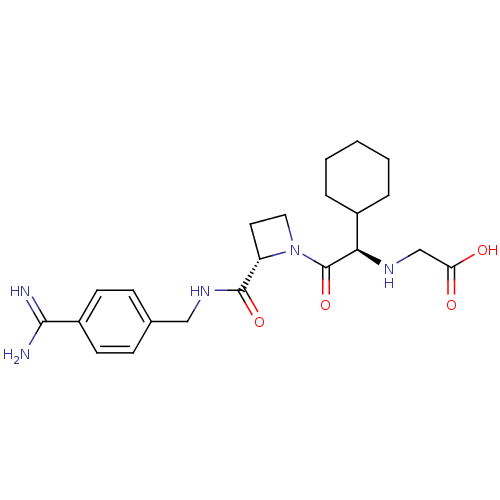 (Exanta | Melagatran | US11584714, Compound 999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118737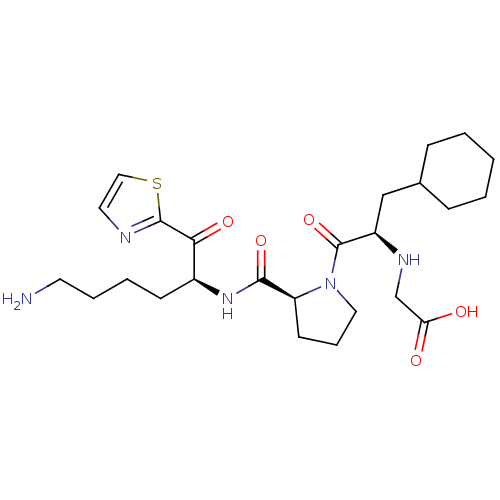 ((2-{2-[5-Amino-1-(thiazole-2-carbonyl)-pentylcarba...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118721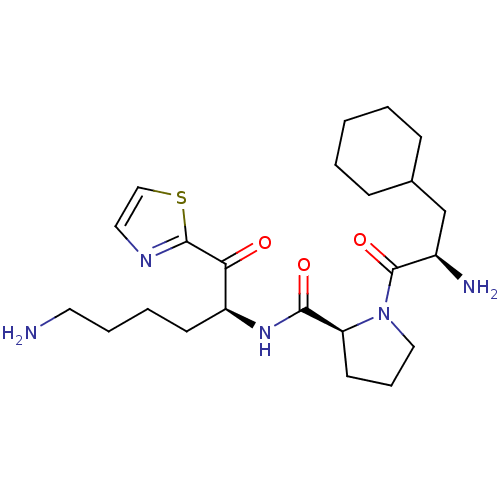 ((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118722 (2-Amino-N-{[5-amino-1-(thiazole-2-carbonyl)-pentyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118725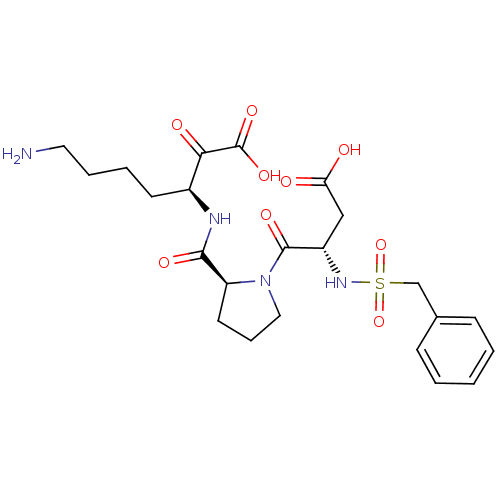 (7-Amino-3-{[1-(3-carboxy-2-phenylmethanesulfonylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038001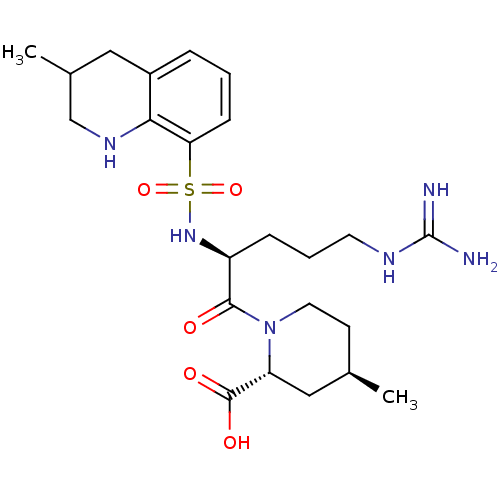 ((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118734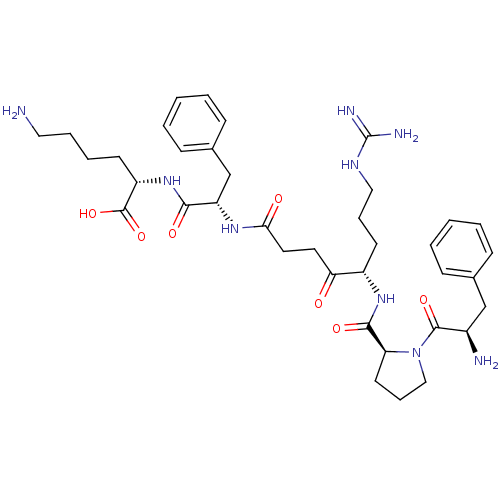 (6-Amino-2-[2-(5-{[1-(2-amino-3-phenyl-propionyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118724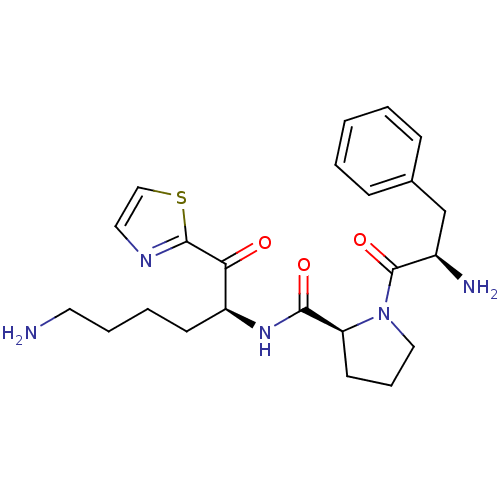 ((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118716 ((S)-1-(3,3-diphenyl-propionyl)-pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118715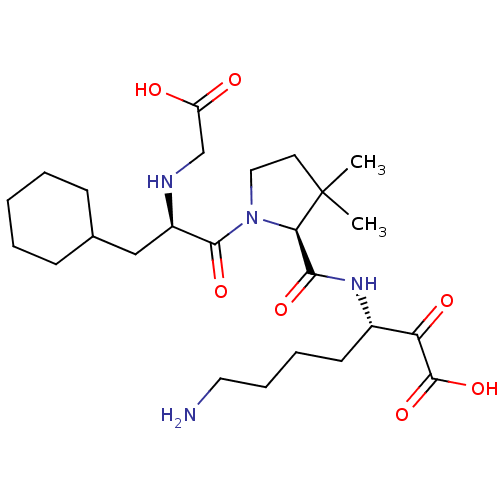 (7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118714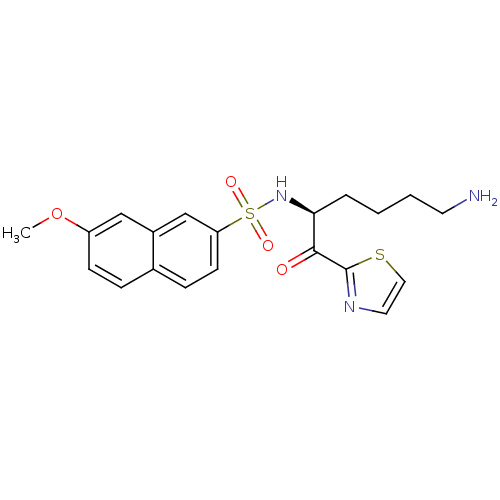 (7-Methoxy-naphthalene-2-sulfonic acid [5-amino-1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118733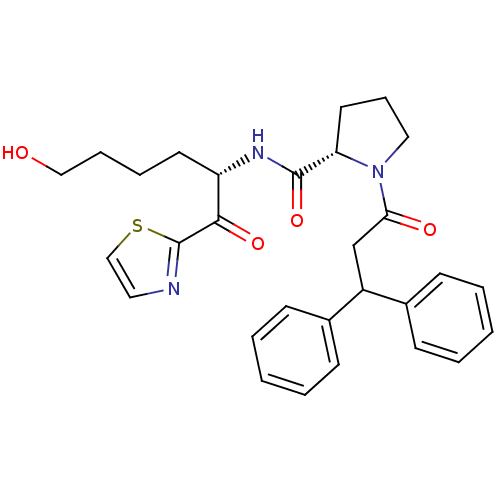 (1-(3,3-Diphenyl-propionyl)-pyrrolidine-2-carboxyli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118726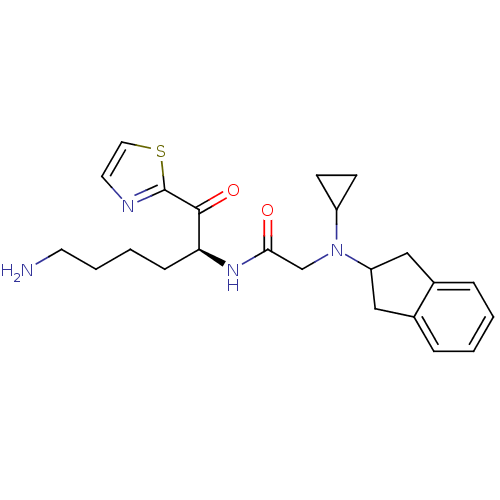 (CHEMBL334701 | N-[5-Amino-1-(thiazole-2-carbonyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50118736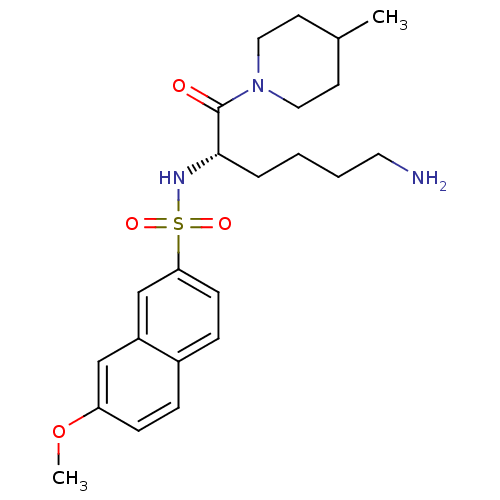 (7-Methoxy-naphthalene-2-sulfonic acid [5-amino-1-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against thrombin. | J Med Chem 45: 4419-32 (2002) BindingDB Entry DOI: 10.7270/Q2SX6DZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50233026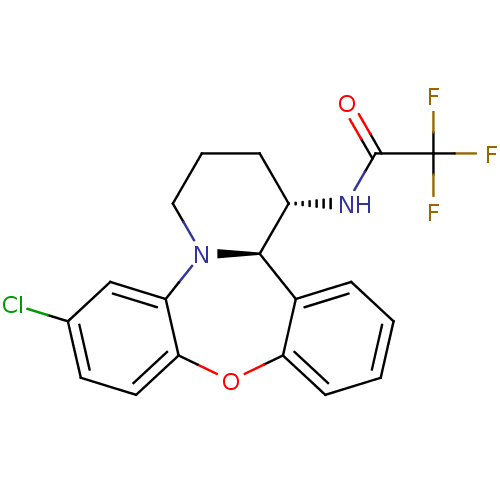 (CHEMBL403138 | N-((1S,13bR)-6-chloro-1,3,4,13b-tet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
N.V. Organon Curated by ChEMBL | Assay Description Inhibition of progesterone receptor expressed in CHO cells by luciferase assay | Bioorg Med Chem Lett 18: 1461-7 (2008) Article DOI: 10.1016/j.bmcl.2007.12.065 BindingDB Entry DOI: 10.7270/Q2BK1C3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267959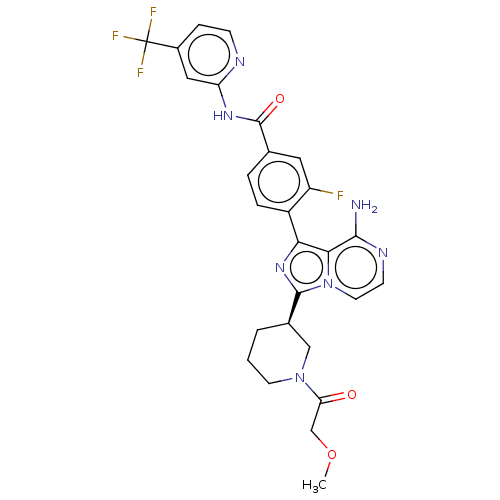 (4-(8-amino-3-{(3R)-1-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267960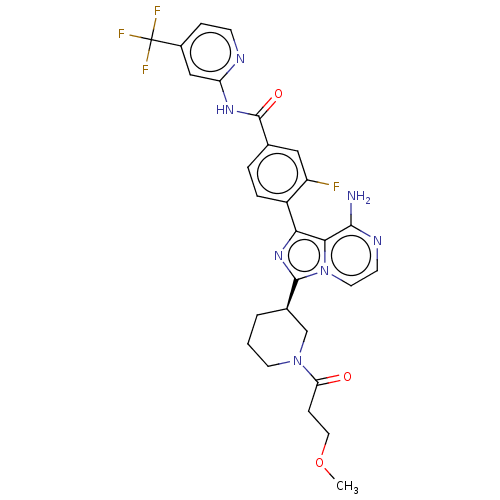 (4-{8-amino-3-[(3R)-1-(methoxyacetyl)piperidin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267886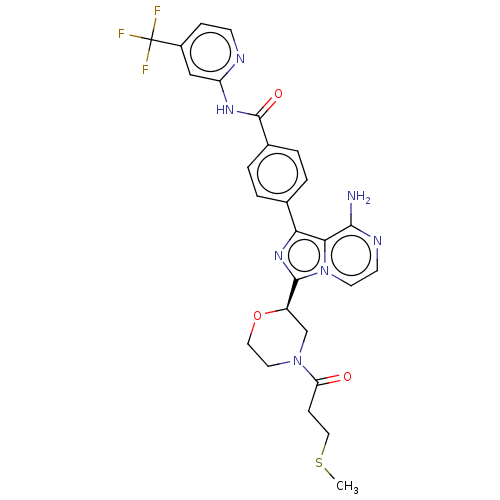 (4-(8-amino-3-{(2R)-4-[(1-methylazetidin-3-yl)carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267887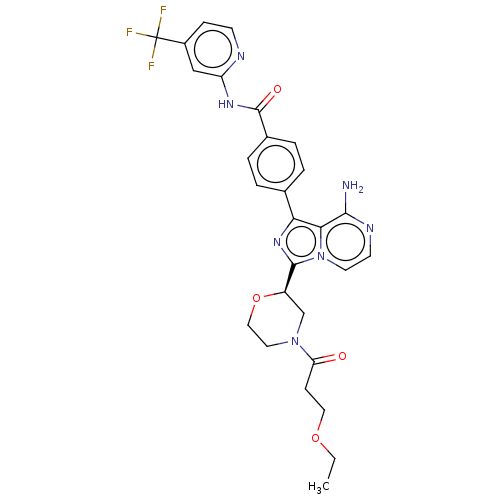 (4-(8-amino-3-{(2R)-4-[3-(methylsulfanyl)propanoyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267888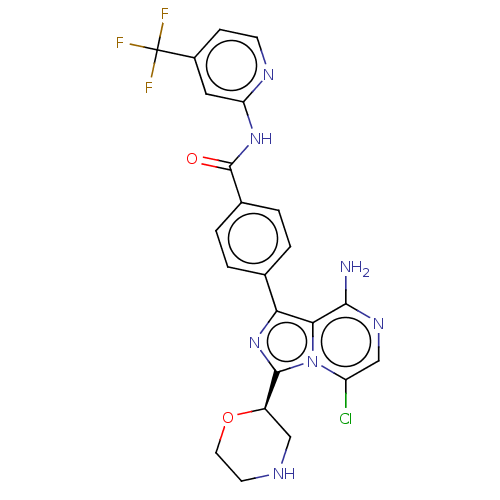 (4-{8-amino-3-[(2R)-4-(3-ethoxypropanoyl)morpholin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267889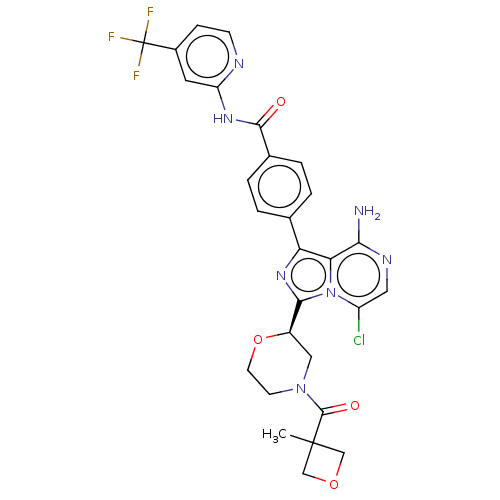 (4-{8-amino-5-chloro-3-[(2R)-morpholin-2-yl]imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267891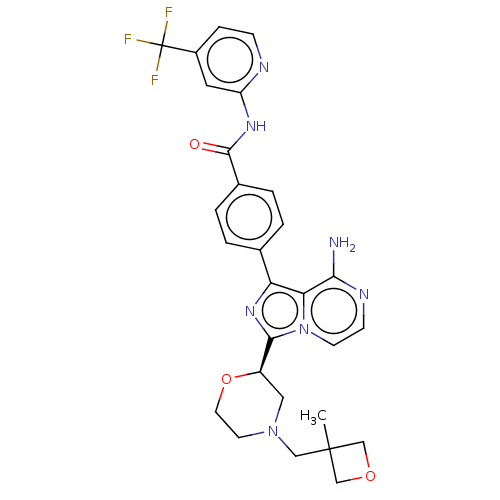 (4-(8-amino-5-methyl-3-{(2R)-4-[(3-methyloxetan-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267892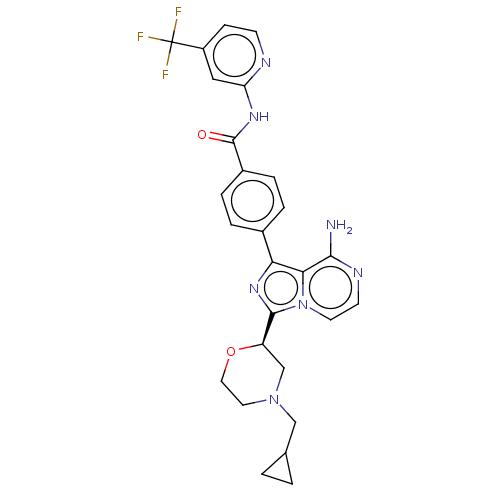 (4-(8-amino-3-{(2R)-4-[(3-methyloxetan-3-yl)methyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267893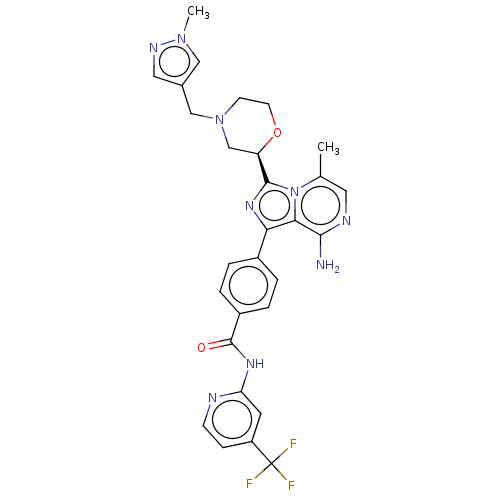 (4-{8-amino-3-[(2R)-4-(cyclopropylmethyl)morpholin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267894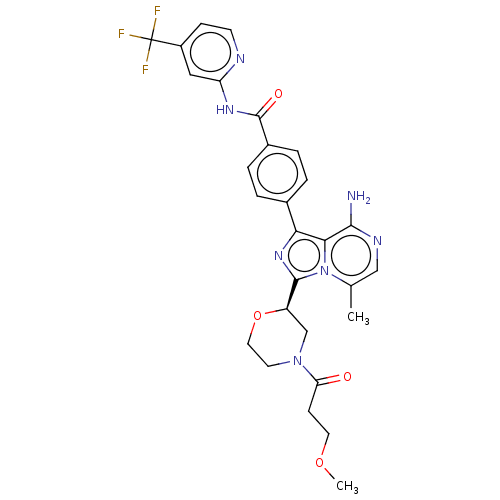 (4-(8-amino-5-methyl-3-{(2R)-4-[(1-methyl-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267895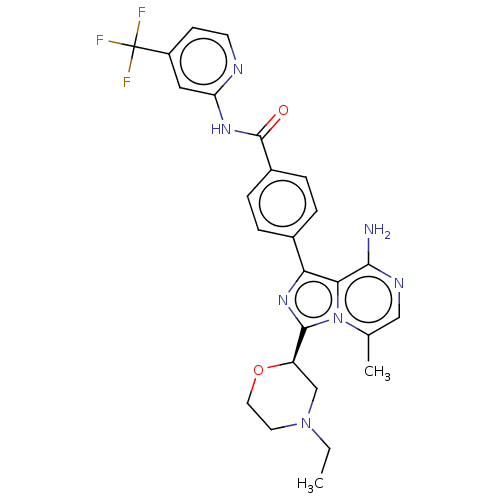 (4-{8-amino-3-[(2R)-4-(3-methoxypropanoyl)morpholin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267896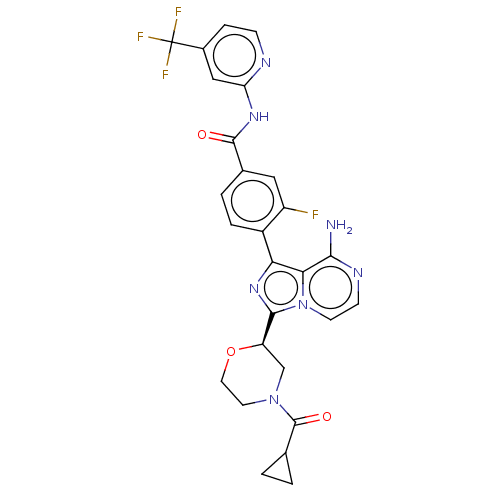 (4-{8-amino-3-[(2R)-4-ethylmorpholin-2-yl]-5-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267897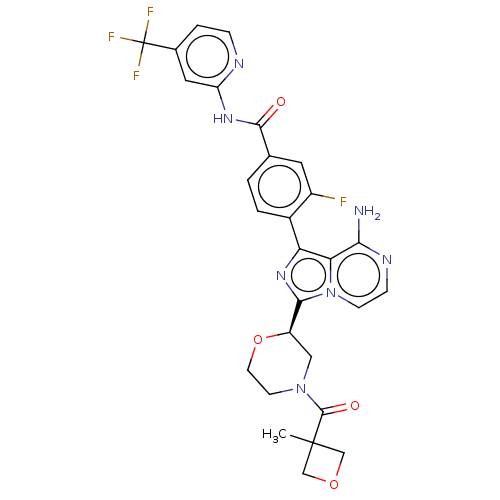 (4-{8-amino-3-[(2R)-4-(cyclopropylcarbonyl)morpholi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267898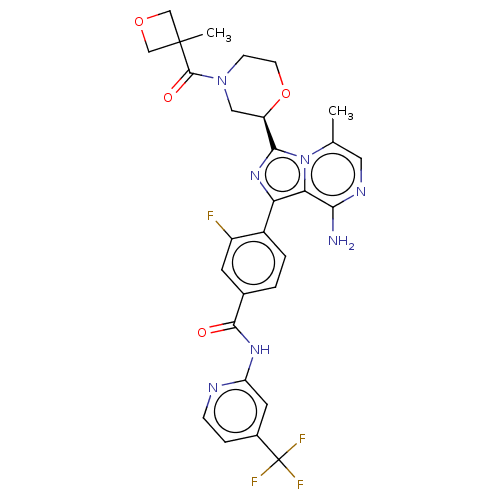 (4-(8-amino-3-{(2R)-4-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267899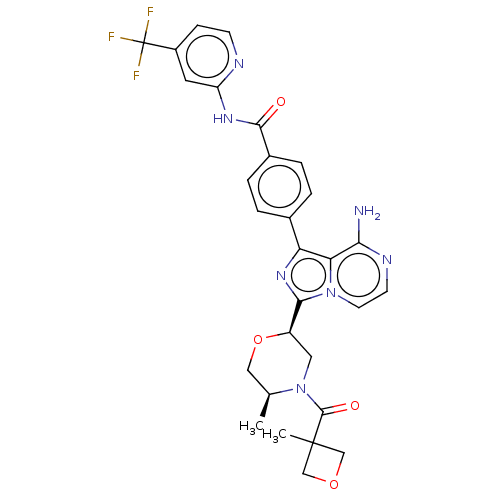 (4-(8-amino-5-methyl-3-{(2R)-4-[(3-methyloxetan-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267900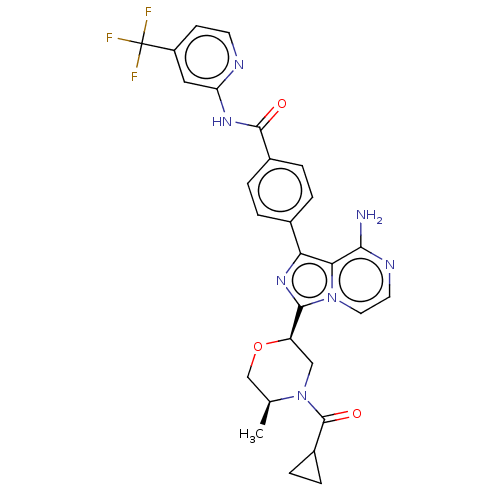 (4-(8-amino-3-{(2R,5S)-5-methyl-4-[(3-methyloxetan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267901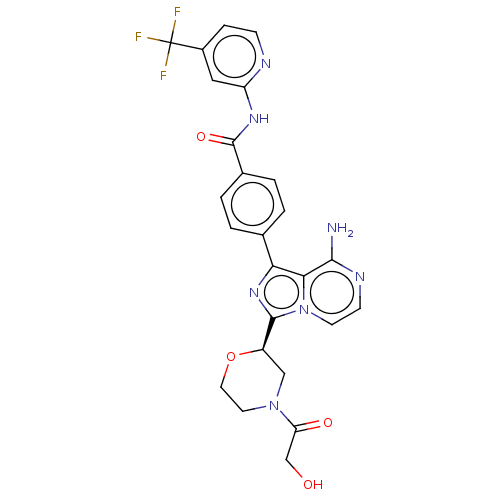 (4-{8-amino-3-[(2R,5S)-4-(cyclopropylcarbonyl)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267902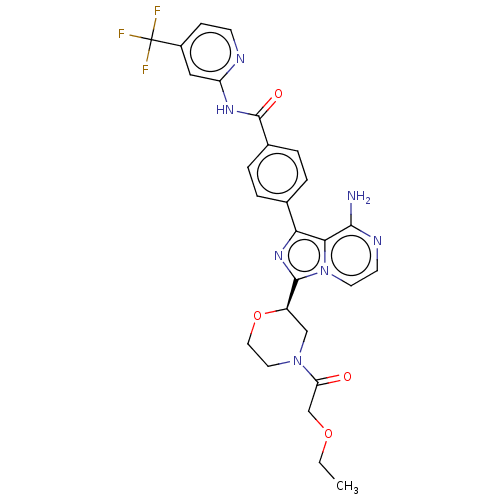 (4-{8-amino-3-[(2R)-4-(hydroxyacetyl)morpholin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267903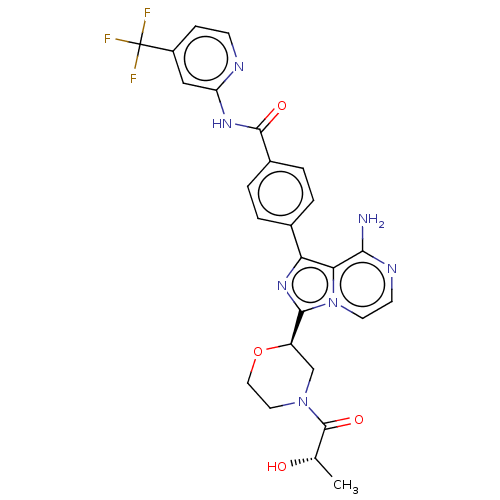 (4-{8-amino-3-[(2R)-4-(ethoxyacetyl)morpholin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267904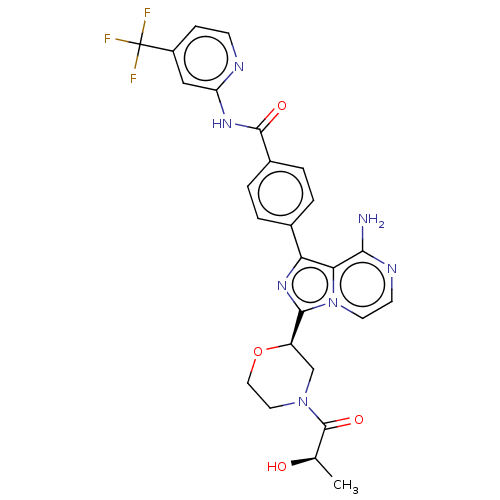 (4-(8-amino-3-{(2R)-4-[(2S)-2-hydroxypropanoyl]morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267905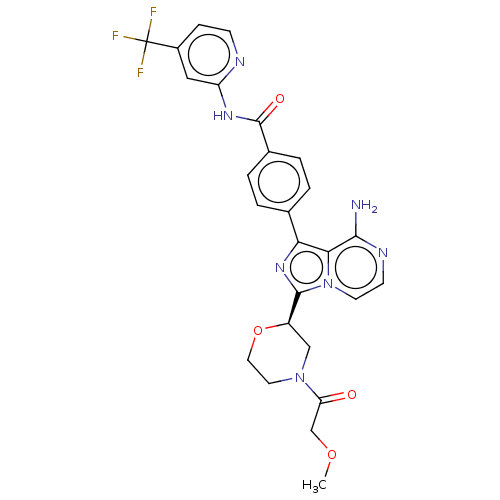 (4-(8-amino-3-{(2R)-4-[(2R)-2-hydroxypropanoyl]morp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2552 total ) | Next | Last >> |