

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434850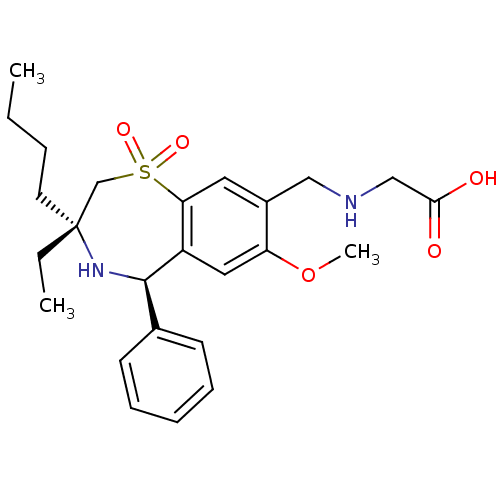 (CHEMBL2387397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125066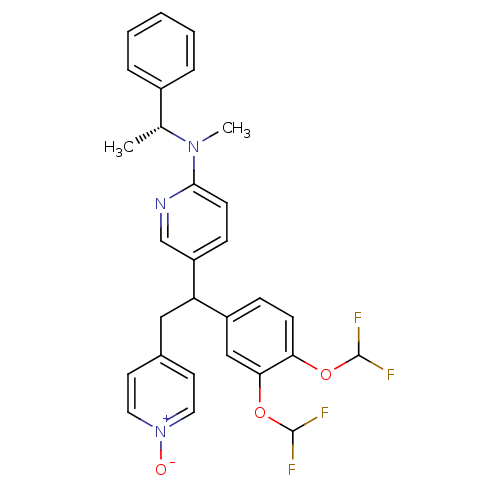 (CHEMBL165889 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434850 (CHEMBL2387397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434849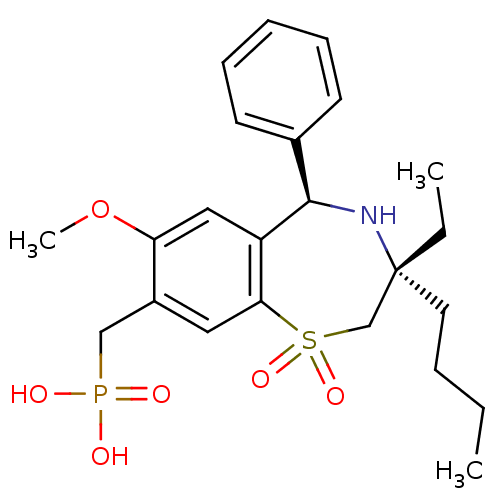 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125065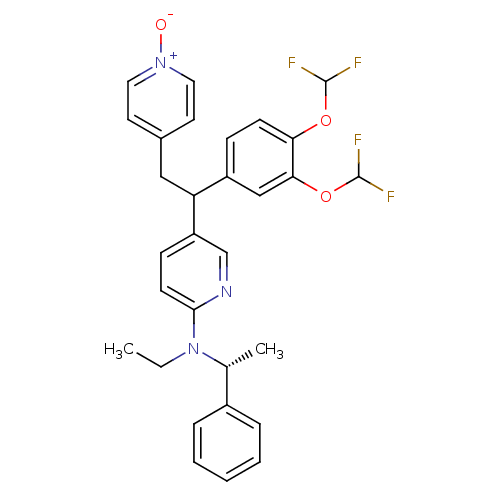 (CHEMBL165687 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125062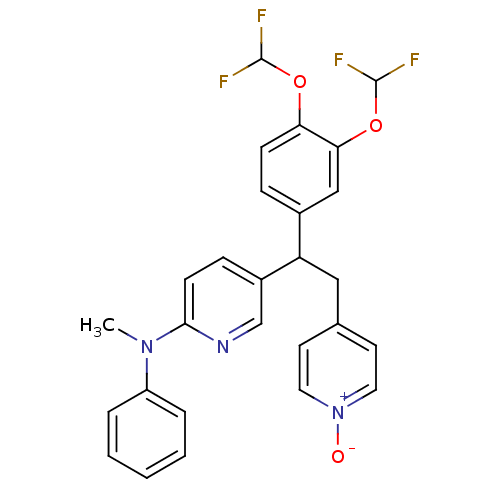 (CHEMBL162485 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125057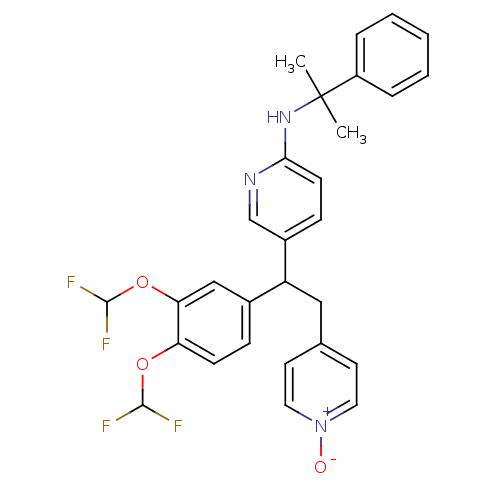 (CHEMBL351187 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434846 (CHEMBL2387399 | US9040518, 35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50049041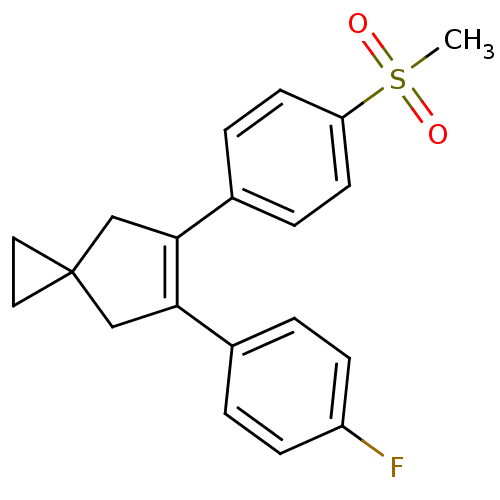 (5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125063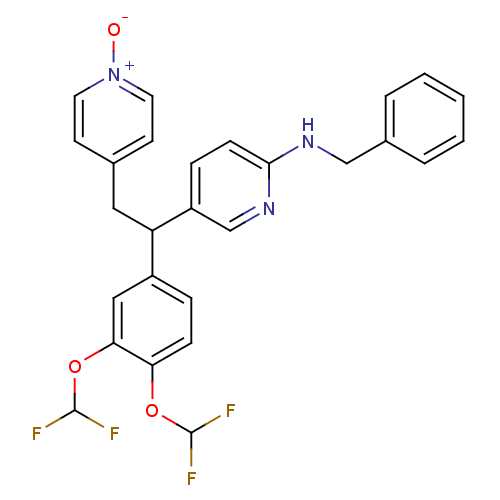 (Benzyl-{5-[1-(3,4-bis-difluoromethoxy-phenyl)-2-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125064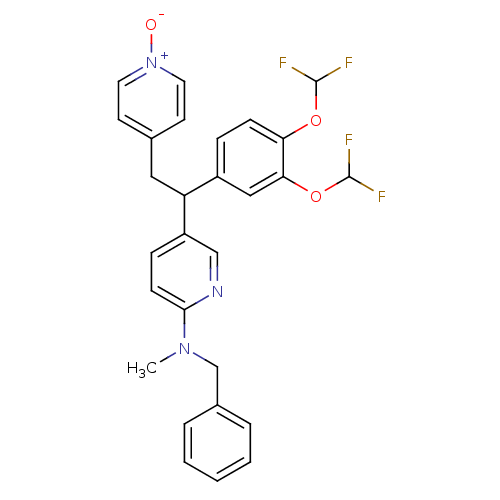 (Benzyl-{5-[1-(3,4-bis-difluoromethoxy-phenyl)-2-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288289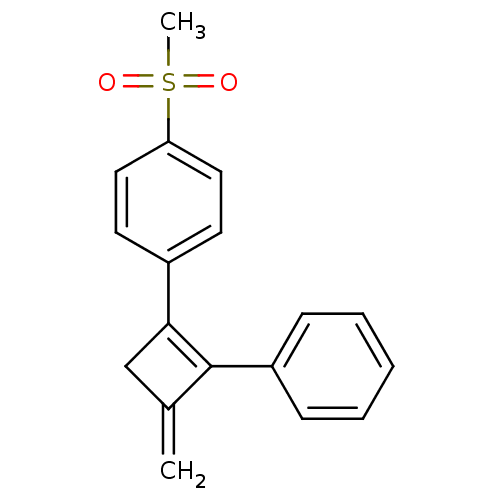 (1-Methanesulfonyl-4-(3-methylene-2-phenyl-cyclobut...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50119355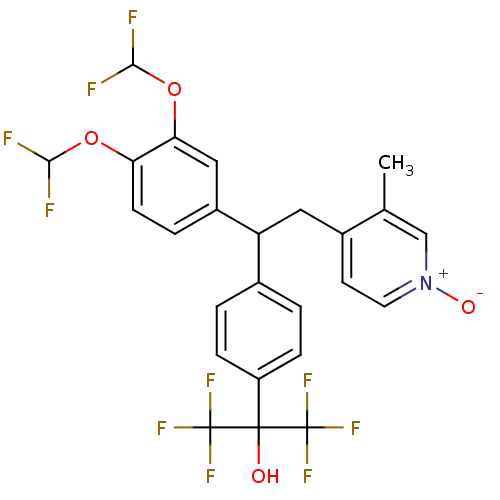 (2-{4-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125074 (CHEMBL444865 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125057 (CHEMBL351187 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125072 (CHEMBL166299 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM47370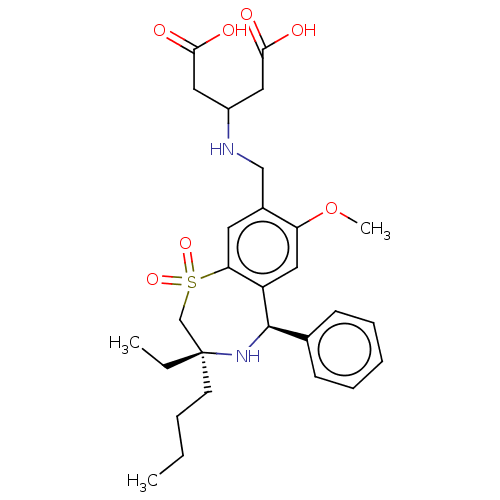 (BDBM50434858 | US9040518, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434847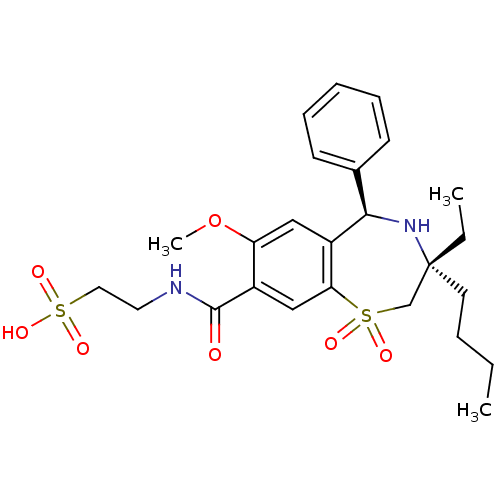 (CHEMBL2387421 | US9040518, 3) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434848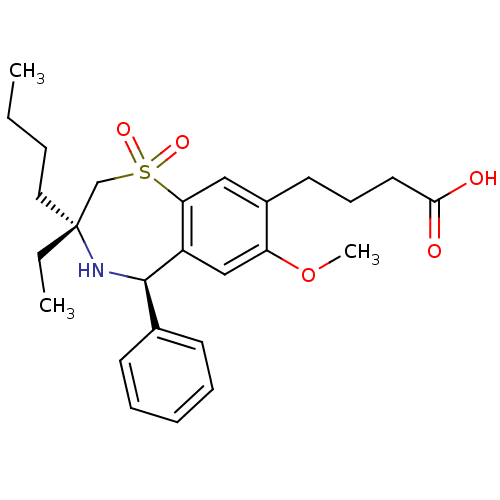 (CHEMBL2387520) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM47370 (BDBM50434858 | US9040518, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600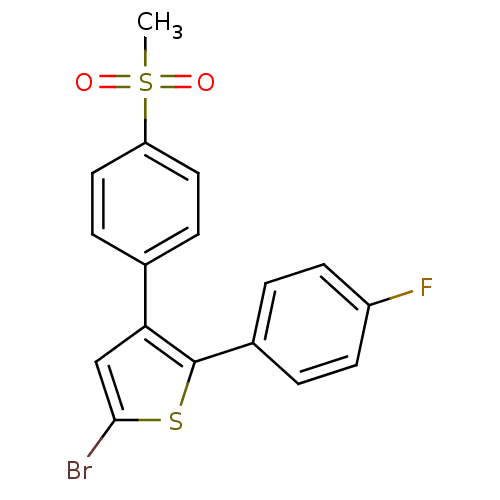 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288292 (1-(4,4-Dimethyl-3-methylene-2-phenyl-cyclobut-1-en...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125076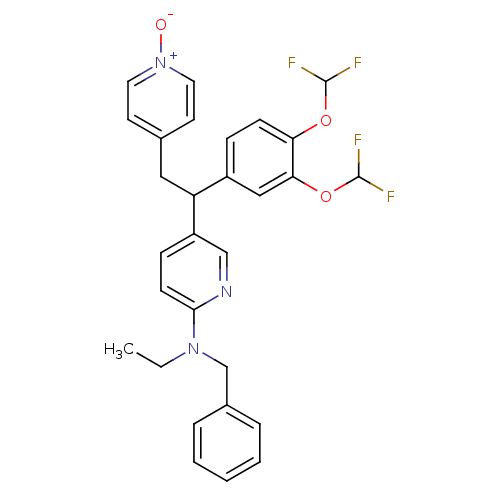 (Benzyl-{5-[1-(3,4-bis-difluoromethoxy-phenyl)-2-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434846 (CHEMBL2387399 | US9040518, 35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125073 (CHEMBL164199 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288291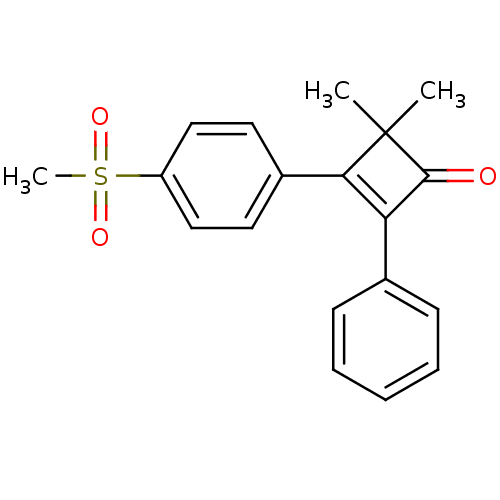 (3-(4-Methanesulfonyl-phenyl)-4,4-dimethyl-2-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50134411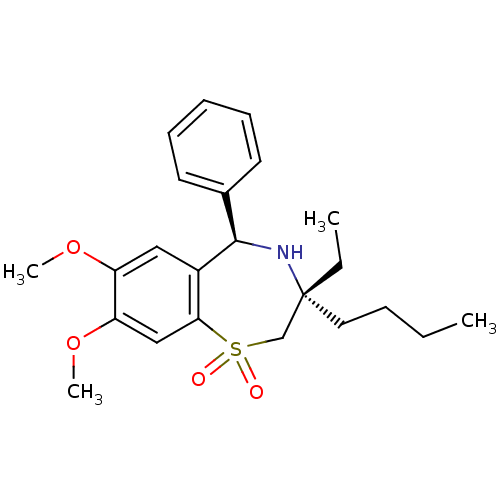 ((7R,9R)-7-Butyl-7-ethyl-2,3-dimethoxy-9-phenyl-6,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434850 (CHEMBL2387397) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434868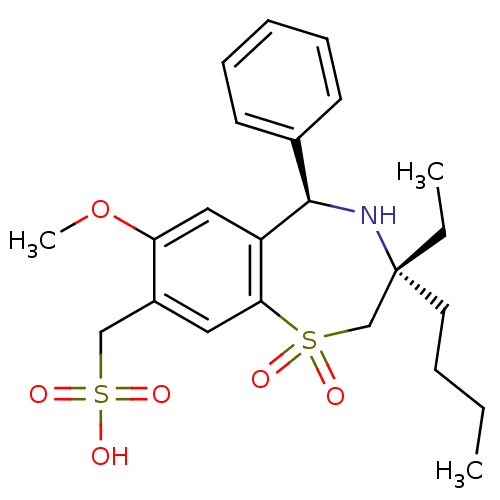 (CHEMBL2387521) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434867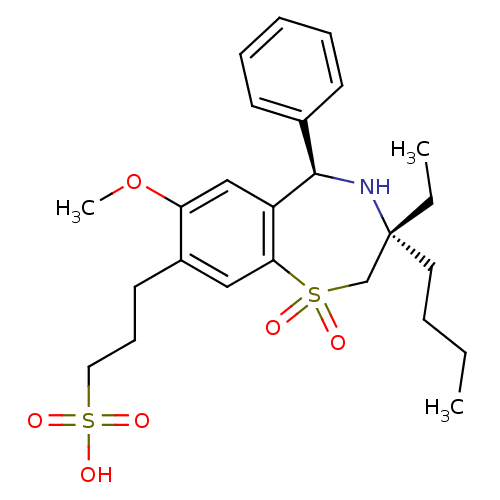 (CHEMBL2387522 | US9040518, 20) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434869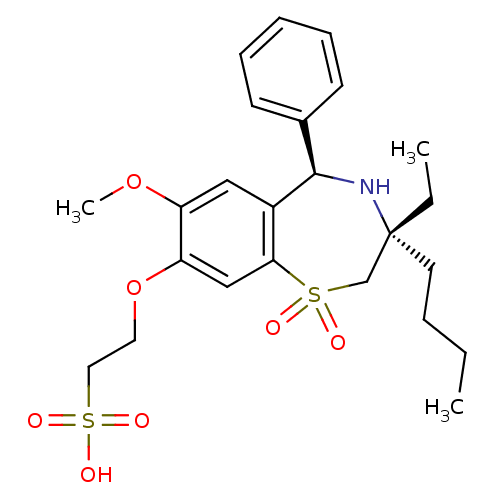 (CHEMBL2387527) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50113425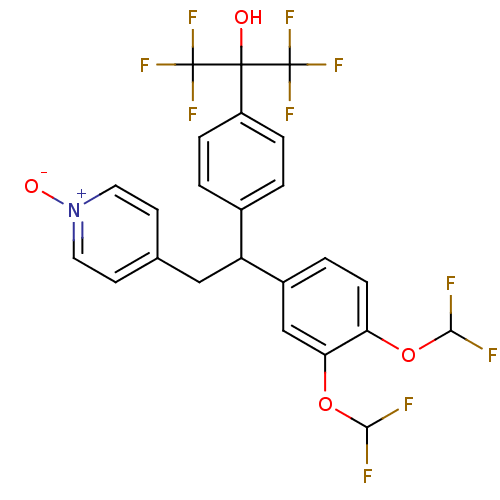 (2-{4-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288287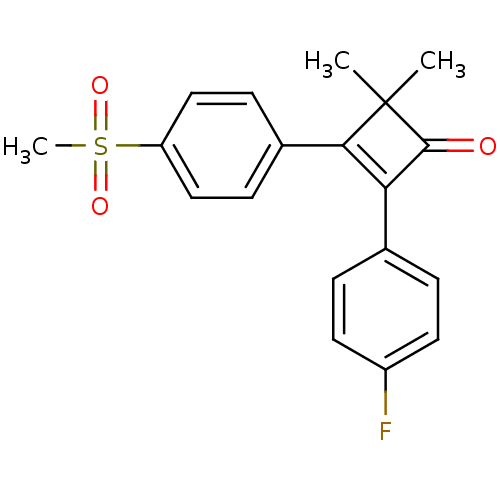 (2-(4-Fluoro-phenyl)-3-(4-methanesulfonyl-phenyl)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125067 (CHEMBL351007 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434866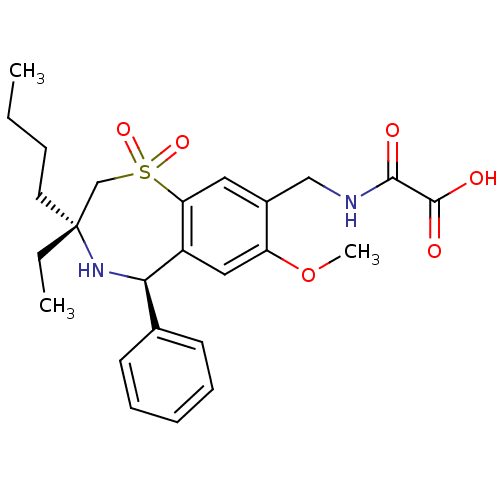 (CHEMBL2387510 | US9040518, 63) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125070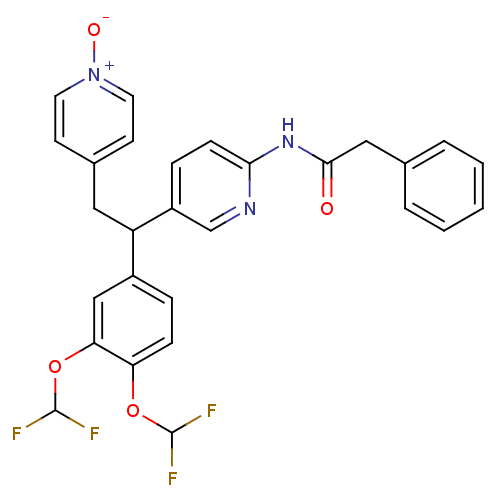 (CHEMBL423252 | N-{5-[1-(3,4-Bis-difluoromethoxy-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125060 (1-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434865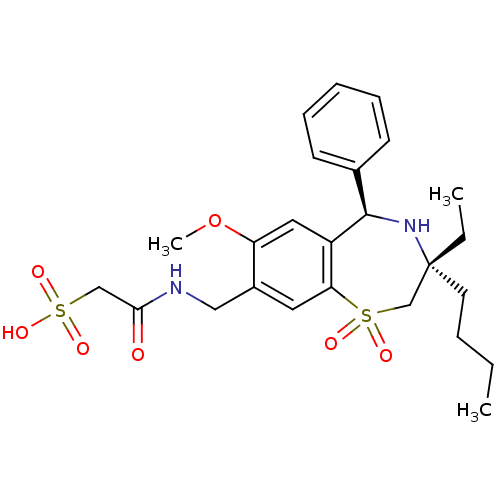 (CHEMBL2387511 | US9040518, 37) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125068 (Benzyl-{5-[1-(3,4-bis-difluoromethoxy-phenyl)-2-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434864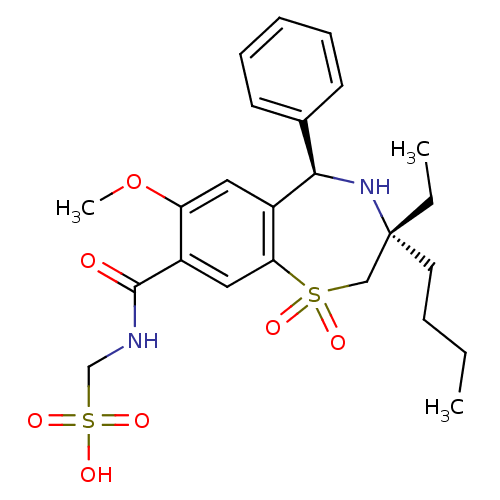 (CHEMBL2387420 | US9040518, 2) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50288294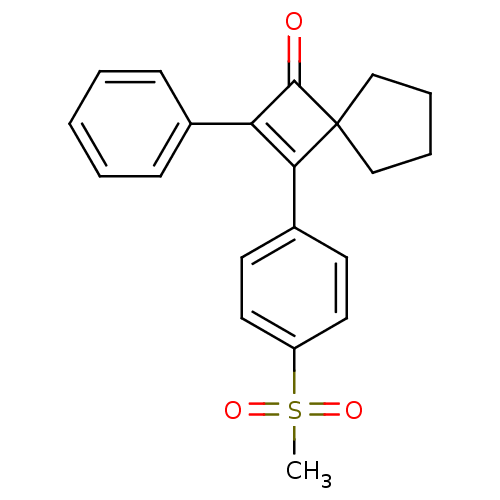 (3-(4-Methanesulfonyl-phenyl)-2-phenyl-spiro[3.4]oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGE-2 production by arachidonic acid-stimulated CHO cells expressing human Prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 6: 2677-2682 (1996) Article DOI: 10.1016/S0960-894X(96)00501-X BindingDB Entry DOI: 10.7270/Q2RB74M8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125059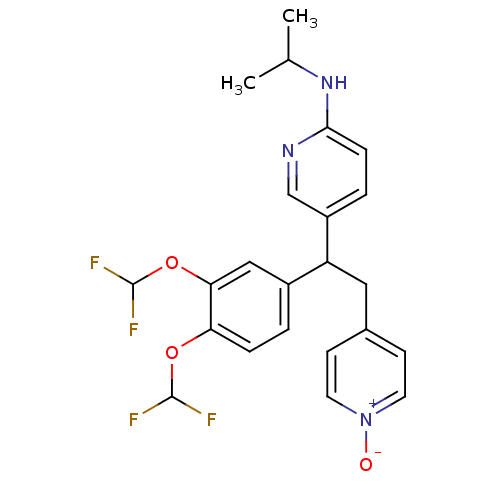 (CHEMBL163311 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434863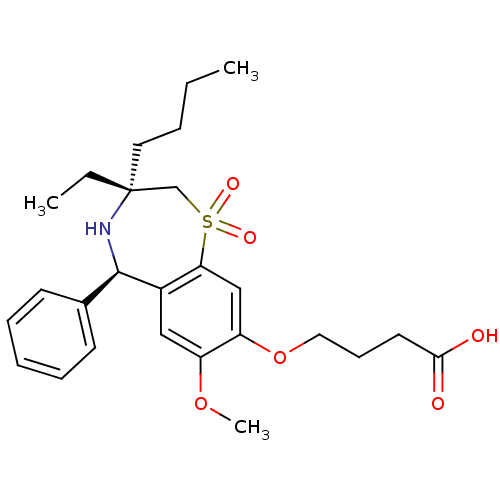 (CHEMBL2387526) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125058 (CHEMBL162870 | N-{5-[1-(3,4-Bis-difluoromethoxy-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434862 (CHEMBL2387519) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125071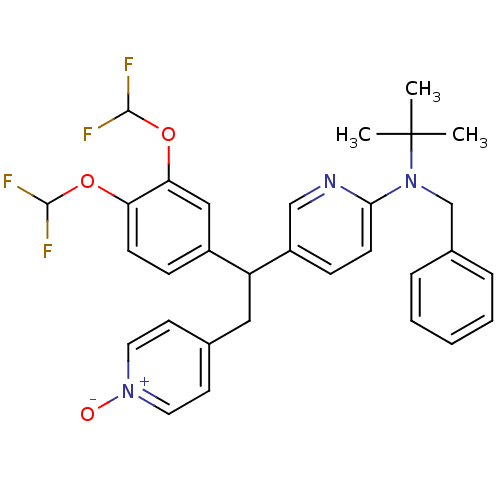 (Benzyl-{5-[1-(3,4-bis-difluoromethoxy-phenyl)-2-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50134411 ((7R,9R)-7-Butyl-7-ethyl-2,3-dimethoxy-9-phenyl-6,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50125057 (CHEMBL351187 | {5-[1-(3,4-Bis-difluoromethoxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... | Bioorg Med Chem Lett 13: 741-4 (2003) BindingDB Entry DOI: 10.7270/Q2PG1R3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |