 Found 1994 hits with Last Name = 'therrien' and Initial = 'e'
Found 1994 hits with Last Name = 'therrien' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50301573
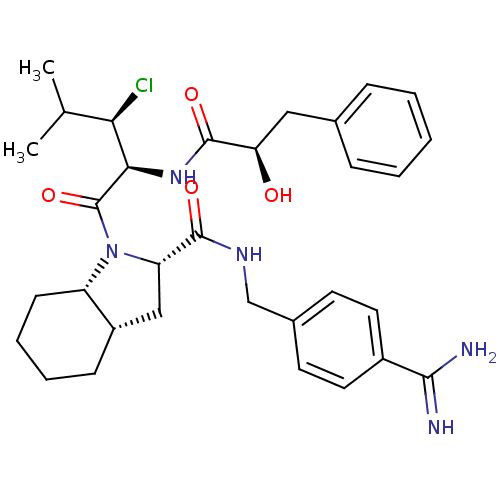
((2S,3aS,7aS)-N-(4-carbamimidoylbenzyl)-1-((2S,3R)-...)Show SMILES CC(C)[C@@H](Cl)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1[C@@H](C[C@@H]2CCCC[C@H]12)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C32H42ClN5O4/c1-19(2)27(33)28(37-31(41)26(39)16-20-8-4-3-5-9-20)32(42)38-24-11-7-6-10-23(24)17-25(38)30(40)36-18-21-12-14-22(15-13-21)29(34)35/h3-5,8-9,12-15,19,23-28,39H,6-7,10-11,16-18H2,1-2H3,(H3,34,35)(H,36,40)(H,37,41)/t23-,24-,25-,26+,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301562
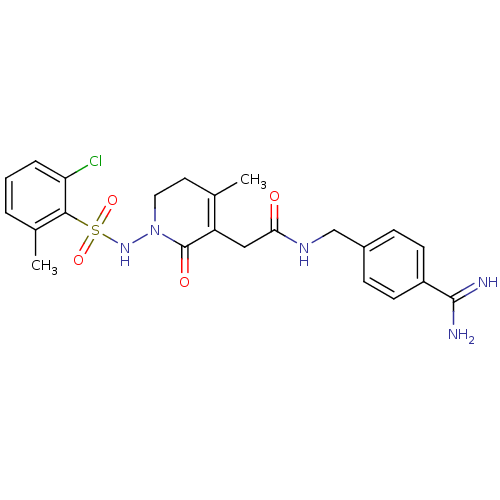
(CHEMBL584260 | N-(4-carbamimidoylbenzyl)-2-(1-(2-c...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1c(C)cccc1Cl |c:1| Show InChI InChI=1S/C23H26ClN5O4S/c1-14-10-11-29(28-34(32,33)21-15(2)4-3-5-19(21)24)23(31)18(14)12-20(30)27-13-16-6-8-17(9-7-16)22(25)26/h3-9,28H,10-13H2,1-2H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301571
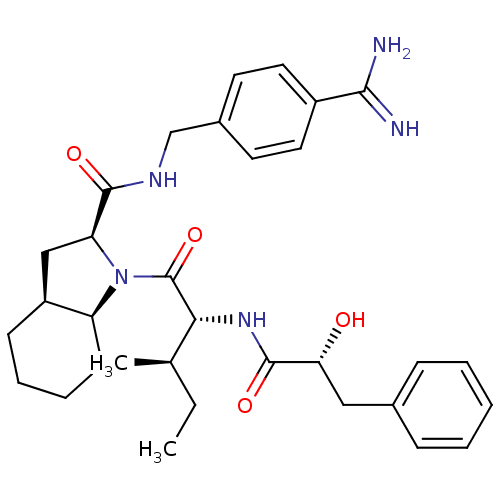
((2S,3aS,7aS)-N-(4-carbamimidoylbenzyl)-1-((2R,3R)-...)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C32H43N5O4/c1-3-20(2)28(36-31(40)27(38)17-21-9-5-4-6-10-21)32(41)37-25-12-8-7-11-24(25)18-26(37)30(39)35-19-22-13-15-23(16-14-22)29(33)34/h4-6,9-10,13-16,20,24-28,38H,3,7-8,11-12,17-19H2,1-2H3,(H3,33,34)(H,35,39)(H,36,40)/t20-,24+,25+,26+,27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301572

((2S,3aS,7aS)-N-(4-carbamimidoylbenzyl)-1-((R)-3,3-...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2C[C@@H]3CCCC[C@@H]3N2C(=O)[C@H](NC(=O)[C@H](O)Cc2ccccc2)C(C2CCCCC2)C2CCCCC2)cc1 |r| Show InChI InChI=1S/C41H57N5O4/c42-38(43)31-22-20-28(21-23-31)26-44-39(48)34-25-32-18-10-11-19-33(32)46(34)41(50)37(45-40(49)35(47)24-27-12-4-1-5-13-27)36(29-14-6-2-7-15-29)30-16-8-3-9-17-30/h1,4-5,12-13,20-23,29-30,32-37,47H,2-3,6-11,14-19,24-26H2,(H3,42,43)(H,44,48)(H,45,49)/t32-,33-,34-,35+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301574

(CHEMBL568990 | Chlorodysinosin A)Show SMILES [#6]-[#8]-[#6@H](-[#6]-[#8]S([#8-])(=O)=O)-[#6](=O)-[#7]-[#6@H](-[#6@H](Cl)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6@H](-[#8])-[#6@@H](-[#8])-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6]-[#6]-1=[#6]-[#6]\[#7+](-[#6]-1)=[#6](\[#7])-[#7] |r,t:38| Show InChI InChI=1S/C26H43ClN6O10S/c1-13(2)21(27)22(31-24(37)20(42-3)12-43-44(39,40)41)25(38)33-16-10-19(35)18(34)9-15(16)8-17(33)23(36)30-6-4-14-5-7-32(11-14)26(28)29/h5,13,15-22,34-35H,4,6-12H2,1-3H3,(H6,28,29,30,31,36,37,39,40,41)/t15-,16+,17+,18+,19+,20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301559

(CHEMBL569249 | N-(4-carbamimidoylbenzyl)-2-(1-(2,5...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1cc(C)ccc1C |c:1| Show InChI InChI=1S/C24H29N5O4S/c1-15-4-5-17(3)21(12-15)34(32,33)28-29-11-10-16(2)20(24(29)31)13-22(30)27-14-18-6-8-19(9-7-18)23(25)26/h4-9,12,28H,10-11,13-14H2,1-3H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50115202
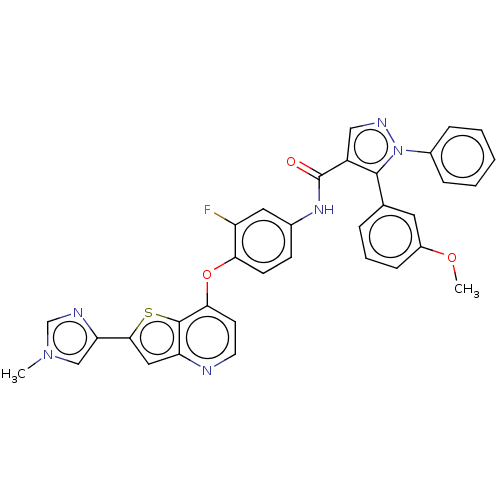
(CHEMBL3608550)Show SMILES COc1cccc(c1)-c1c(cnn1-c1ccccc1)C(=O)Nc1ccc(Oc2ccnc3cc(sc23)-c2cn(C)cn2)c(F)c1 Show InChI InChI=1S/C34H25FN6O3S/c1-40-19-28(37-20-40)31-17-27-33(45-31)30(13-14-36-27)44-29-12-11-22(16-26(29)35)39-34(42)25-18-38-41(23-8-4-3-5-9-23)32(25)21-7-6-10-24(15-21)43-2/h3-20H,1-2H3,(H,39,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human c-Met kinase domain |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50277188

(CHEMBL473270 | N1-(biphenyl-3-yl)-N8-hydroxyoctane...)Show InChI InChI=1S/C20H24N2O3/c23-19(13-6-1-2-7-14-20(24)22-25)21-18-12-8-11-17(15-18)16-9-4-3-5-10-16/h3-5,8-12,15,25H,1-2,6-7,13-14H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal histidine-tagged HDAC6 expressed in baculovirus by fluorimetry |
Bioorg Med Chem Lett 19: 336-40 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.081
BindingDB Entry DOI: 10.7270/Q2416WXB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50277188

(CHEMBL473270 | N1-(biphenyl-3-yl)-N8-hydroxyoctane...)Show InChI InChI=1S/C20H24N2O3/c23-19(13-6-1-2-7-14-20(24)22-25)21-18-12-8-11-17(15-18)16-9-4-3-5-10-16/h3-5,8-12,15,25H,1-2,6-7,13-14H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal FLAG-tagged HDAC1 expressed in baculovirus by fluorimetry |
Bioorg Med Chem Lett 19: 336-40 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.081
BindingDB Entry DOI: 10.7270/Q2416WXB |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM16055

((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCCCC)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C34H63N5O6S/c1-10-11-16-35-34(45)29(22(6)7)39-31(42)25-14-12-13-24(25)30(41)27(18-20(2)3)38-32(43)26(15-17-46-9)37-33(44)28(19-21(4)5)36-23(8)40/h20-22,24-30,41H,10-19H2,1-9H3,(H,35,45)(H,36,40)(H,37,44)(H,38,43)(H,39,42)/t24-,25-,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50317323
(CHEMBL1094718 | N-(3-fluoro-4-(2-(1-methyl-1H-imid...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-14-21(34-15-37)24-12-20-25(41-24)23(9-10-33-20)40-22-8-7-16(11-19(22)29)36-27(39)18-13-35-38(26(18)28(30,31)32)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc. / ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3706-10 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.034
BindingDB Entry DOI: 10.7270/Q2SJ1NDW |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50115203
(CHEMBL3608551)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4-c4cccnc4)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C32H22FN7O2S/c1-39-18-26(36-19-39)29-15-25-31(43-29)28(11-13-35-25)42-27-10-9-21(14-24(27)33)38-32(41)23-17-37-40(22-7-3-2-4-8-22)30(23)20-6-5-12-34-16-20/h2-19H,1H3,(H,38,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human c-Met kinase domain |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50091030
(CHEMBL3582033)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4ccn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C13H21N3/c1-2-5-16-6-3-4-10-7-12-11(8-13(10)16)9-14-15-12/h9-10,13H,2-8H2,1H3,(H,14,15)/p+1/t10-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON (unknown origin) transfected in 293T cells assessed as autophosporylated level by ELISA |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM16054

((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C)C(=O)NCCCC)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C32H59N5O6S/c1-9-10-15-33-29(40)21(6)34-30(41)24-13-11-12-23(24)28(39)26(17-19(2)3)37-31(42)25(14-16-44-8)36-32(43)27(18-20(4)5)35-22(7)38/h19-21,23-28,39H,9-18H2,1-8H3,(H,33,40)(H,34,41)(H,35,38)(H,36,43)(H,37,42)/t21-,23+,24+,25-,26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM16049
((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C |r| Show InChI InChI=1S/C43H66N8O14/c1-21(2)18-29(48-41(62)30(20-32(45)52)49-42(63)35(22(3)4)51-39(60)27(44)14-16-33(53)54)36(57)25-12-9-13-26(25)38(59)46-23(5)37(58)47-28(15-17-34(55)56)40(61)50-31(43(64)65)19-24-10-7-6-8-11-24/h6-8,10-11,21-23,25-31,35-36,57H,9,12-20,44H2,1-5H3,(H2,45,52)(H,46,59)(H,47,58)(H,48,62)(H,49,63)(H,50,61)(H,51,60)(H,53,54)(H,55,56)(H,64,65)/t23-,25+,26+,27-,28-,29-,30-,31-,35-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16060
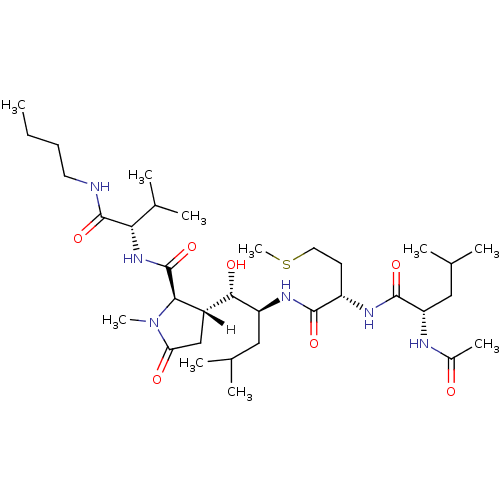
((2R,3R)-3-[(1S,2S)-2-[[(2S)-2-[[(2S)-2-acetamido-4...)Show SMILES [H][C@]1(CC(=O)N(C)[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCCCC)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C34H62N6O7S/c1-11-12-14-35-33(46)28(21(6)7)39-34(47)29-23(18-27(42)40(29)9)30(43)25(16-19(2)3)38-31(44)24(13-15-48-10)37-32(45)26(17-20(4)5)36-22(8)41/h19-21,23-26,28-30,43H,11-18H2,1-10H3,(H,35,46)(H,36,41)(H,37,45)(H,38,44)(H,39,47)/t23-,24+,25+,26+,28+,29-,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16057
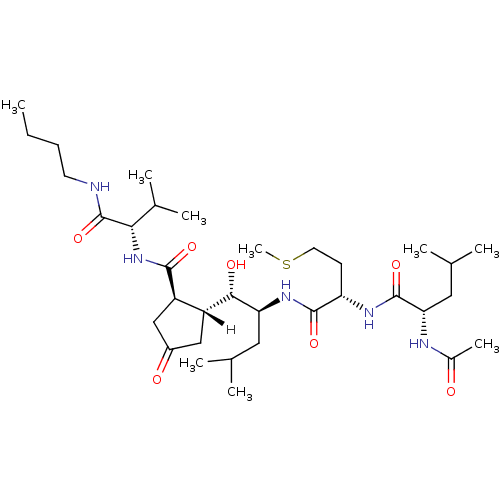
((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...)Show SMILES [H][C@]1(CC(=O)C[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCCCC)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C34H61N5O7S/c1-10-11-13-35-34(46)29(21(6)7)39-31(43)25-18-23(41)17-24(25)30(42)27(15-19(2)3)38-32(44)26(12-14-47-9)37-33(45)28(16-20(4)5)36-22(8)40/h19-21,24-30,42H,10-18H2,1-9H3,(H,35,46)(H,36,40)(H,37,45)(H,38,44)(H,39,43)/t24-,25-,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50301561

(CHEMBL565328 | N-(4-carbamimidoylbenzyl)-2-(1-(2,5...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1cc(Cl)ccc1Cl |c:1| Show InChI InChI=1S/C22H23Cl2N5O4S/c1-13-8-9-29(28-34(32,33)19-10-16(23)6-7-18(19)24)22(31)17(13)11-20(30)27-12-14-2-4-15(5-3-14)21(25)26/h2-7,10,28H,8-9,11-12H2,1H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301558

(CHEMBL565292 | N-(4-carbamimidoylbenzyl)-2-(1-(3-m...)Show SMILES COc1cccc(c1)S(=O)(=O)NN1CCC(C)=C(CC(=O)NCc2ccc(cc2)C(N)=N)C1=O |t:17| Show InChI InChI=1S/C23H27N5O5S/c1-15-10-11-28(27-34(31,32)19-5-3-4-18(12-19)33-2)23(30)20(15)13-21(29)26-14-16-6-8-17(9-7-16)22(24)25/h3-9,12,27H,10-11,13-14H2,1-2H3,(H3,24,25)(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50317323

(CHEMBL1094718 | N-(3-fluoro-4-(2-(1-methyl-1H-imid...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-14-21(34-15-37)24-12-20-25(41-24)23(9-10-33-20)40-22-8-7-16(11-19(22)29)36-27(39)18-13-35-38(26(18)28(30,31)32)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16048

((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1R...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(O)=O |r| Show InChI InChI=1S/C27H48N6O10/c1-12(2)9-17(19(34)10-14(5)23(38)30-15(6)27(42)43)31-25(40)18(11-20(29)35)32-26(41)22(13(3)4)33-24(39)16(28)7-8-21(36)37/h12-19,22,34H,7-11,28H2,1-6H3,(H2,29,35)(H,30,38)(H,31,40)(H,32,41)(H,33,39)(H,36,37)(H,42,43)/t14-,15+,16+,17+,18+,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50317323

(CHEMBL1094718 | N-(3-fluoro-4-(2-(1-methyl-1H-imid...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-14-21(34-15-37)24-12-20-25(41-24)23(9-10-33-20)40-22-8-7-16(11-19(22)29)36-27(39)18-13-35-38(26(18)28(30,31)32)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc. / ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human c-Met |
Bioorg Med Chem Lett 25: 3706-10 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.034
BindingDB Entry DOI: 10.7270/Q2SJ1NDW |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50317323

(CHEMBL1094718 | N-(3-fluoro-4-(2-(1-methyl-1H-imid...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-14-21(34-15-37)24-12-20-25(41-24)23(9-10-33-20)40-22-8-7-16(11-19(22)29)36-27(39)18-13-35-38(26(18)28(30,31)32)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON (unknown origin) transfected in 293T cells assessed as autophosporylated level by ELISA |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50090969

(CHEMBL3582034)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4ncn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-13-20(34-14-37)23-12-19-25(41-23)22(9-10-33-19)40-21-8-7-16(11-18(21)29)36-27(39)24-26(28(30,31)32)38(15-35-24)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON (unknown origin) transfected in 293T cells assessed as autophosporylated level by ELISA |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50115203

(CHEMBL3608551)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4-c4cccnc4)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C32H22FN7O2S/c1-39-18-26(36-19-39)29-15-25-31(43-29)28(11-13-35-25)42-27-10-9-21(14-24(27)33)38-32(41)23-17-37-40(22-7-3-2-4-8-22)30(23)20-6-5-12-34-16-20/h2-19H,1H3,(H,38,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50317324

(CHEMBL1087984 | N-(4-(6,7-dimethoxyquinolin-4-ylox...)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2cc1OC Show InChI InChI=1S/C28H20F4N4O4/c1-38-24-13-18-21(14-25(24)39-2)33-11-10-22(18)40-23-9-8-16(12-20(23)29)35-27(37)19-15-34-36(26(19)28(30,31)32)17-6-4-3-5-7-17/h3-15H,1-2H3,(H,35,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON (unknown origin) transfected in 293T cells assessed as autophosporylated level by ELISA |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177433
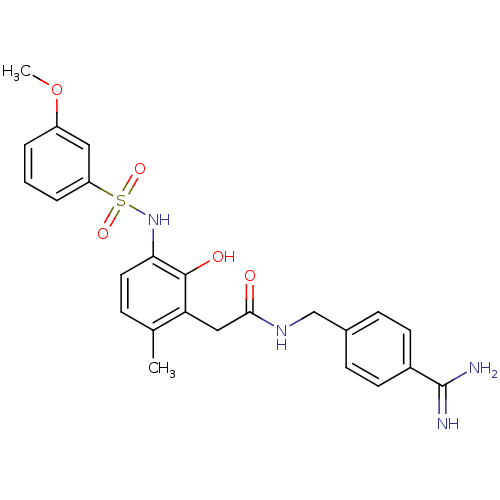
(CHEMBL535160 | N-(4-carbamimidoylbenzyl)-2-(2-hydr...)Show SMILES COc1cccc(c1)S(=O)(=O)Nc1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1O Show InChI InChI=1S/C24H26N4O5S/c1-15-6-11-21(28-34(31,32)19-5-3-4-18(12-19)33-2)23(30)20(15)13-22(29)27-14-16-7-9-17(10-8-16)24(25)26/h3-12,28,30H,13-14H2,1-2H3,(H3,25,26)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16049

((4S)-4-[(2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C |r| Show InChI InChI=1S/C43H66N8O14/c1-21(2)18-29(48-41(62)30(20-32(45)52)49-42(63)35(22(3)4)51-39(60)27(44)14-16-33(53)54)36(57)25-12-9-13-26(25)38(59)46-23(5)37(58)47-28(15-17-34(55)56)40(61)50-31(43(64)65)19-24-10-7-6-8-11-24/h6-8,10-11,21-23,25-31,35-36,57H,9,12-20,44H2,1-5H3,(H2,45,52)(H,46,59)(H,47,58)(H,48,62)(H,49,63)(H,50,61)(H,51,60)(H,53,54)(H,55,56)(H,64,65)/t23-,25+,26+,27-,28-,29-,30-,31-,35-,36-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177436

(CHEMBL557787 | N-(4-carbamimidoyl-benzyl)-2-[2-hyd...)Show SMILES Cc1ccc(NS(=O)(=O)c2cccc3ccccc23)c(O)c1CC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C27H26N4O4S/c1-17-9-14-23(31-36(34,35)24-8-4-6-19-5-2-3-7-21(19)24)26(33)22(17)15-25(32)30-16-18-10-12-20(13-11-18)27(28)29/h2-14,31,33H,15-16H2,1H3,(H3,28,29)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50090968

(CHEMBL3582035)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4nnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C27H17F4N7O2S/c1-37-13-19(33-14-37)22-12-18-24(41-22)21(9-10-32-18)40-20-8-7-15(11-17(20)28)34-26(39)23-25(27(29,30)31)38(36-35-23)16-5-3-2-4-6-16/h2-14H,1H3,(H,34,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON (unknown origin) transfected in 293T cells assessed as autophosporylated level by ELISA |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50119079
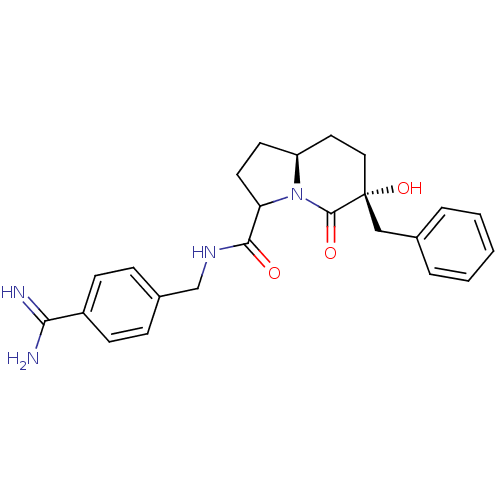
((6R,8aS)-6-Benzyl-6-hydroxy-5-oxo-octahydro-indoli...)Show SMILES NC(=N)c1ccc(CNC(=O)C2CC[C@H]3CC[C@@](O)(Cc4ccccc4)C(=O)N23)cc1 Show InChI InChI=1S/C24H28N4O3/c25-21(26)18-8-6-17(7-9-18)15-27-22(29)20-11-10-19-12-13-24(31,23(30)28(19)20)14-16-4-2-1-3-5-16/h1-9,19-20,31H,10-15H2,(H3,25,26)(H,27,29)/t19-,20?,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Thrombin |
Bioorg Med Chem Lett 12: 2907-11 (2002)
BindingDB Entry DOI: 10.7270/Q2ZS2X22 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50119079

((6R,8aS)-6-Benzyl-6-hydroxy-5-oxo-octahydro-indoli...)Show SMILES NC(=N)c1ccc(CNC(=O)C2CC[C@H]3CC[C@@](O)(Cc4ccccc4)C(=O)N23)cc1 Show InChI InChI=1S/C24H28N4O3/c25-21(26)18-8-6-17(7-9-18)15-27-22(29)20-11-10-19-12-13-24(31,23(30)28(19)20)14-16-4-2-1-3-5-16/h1-9,19-20,31H,10-15H2,(H3,25,26)(H,27,29)/t19-,20?,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Thrombin |
Bioorg Med Chem Lett 12: 2907-11 (2002)
BindingDB Entry DOI: 10.7270/Q2ZS2X22 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50115196

(CHEMBL3608549)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4-c4ccccn4)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C32H22FN7O2S/c1-39-18-26(36-19-39)29-16-25-31(43-29)28(12-14-35-25)42-27-11-10-20(15-23(27)33)38-32(41)22-17-37-40(21-7-3-2-4-8-21)30(22)24-9-5-6-13-34-24/h2-19H,1H3,(H,38,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50235535

(CHEMBL559192 | N-(4-carbamimidoyl-benzyl)-2-[4-met...)Show SMILES Cc1ccccc1CCNn1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1=O Show InChI InChI=1S/C25H29N5O2/c1-17-5-3-4-6-20(17)11-13-29-30-14-12-18(2)22(25(30)32)15-23(31)28-16-19-7-9-21(10-8-19)24(26)27/h3-10,12,14,29H,11,13,15-16H2,1-2H3,(H3,26,27)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 18: 1972-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.122
BindingDB Entry DOI: 10.7270/Q2833SV0 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50115201
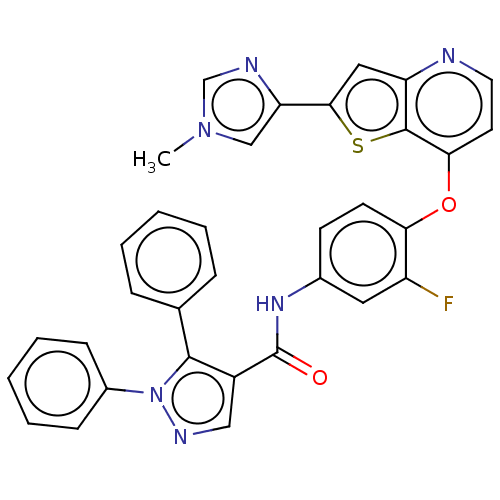
(CHEMBL3608544)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4-c4ccccc4)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C33H23FN6O2S/c1-39-19-27(36-20-39)30-17-26-32(43-30)29(14-15-35-26)42-28-13-12-22(16-25(28)34)38-33(41)24-18-37-40(23-10-6-3-7-11-23)31(24)21-8-4-2-5-9-21/h2-20H,1H3,(H,38,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50115197

(CHEMBL3608547)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4-c4ccccc4F)-c4ccccc4)cc3F)c2s1 |(7.42,-1.7,;6.48,-.91,;6.59,.63,;5.16,1.21,;4.2,.03,;4.99,-1.28,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;-1.02,-3.09,;-2.35,-3.87,;-3.69,-3.1,;-5.02,-3.88,;-5.02,-5.42,;-6.35,-6.19,;-6.34,-7.73,;-5.27,-8.35,;-7.67,-8.51,;-9.06,-7.88,;-10.09,-9.02,;-9.32,-10.35,;-7.81,-10.03,;-6.66,-11.05,;-7.09,-12.53,;-6.02,-13.64,;-4.53,-13.27,;-4.1,-11.79,;-5.17,-10.68,;-4.83,-9.5,;-9.94,-11.76,;-9.13,-13.07,;-9.86,-14.43,;-11.4,-14.47,;-12.21,-13.17,;-11.48,-11.81,;-3.68,-6.18,;-2.35,-5.41,;-1.28,-6.02,;.3,-.77,;1.76,-1.24,)| Show InChI InChI=1S/C33H22F2N6O2S/c1-40-18-27(37-19-40)30-16-26-32(44-30)29(13-14-36-26)43-28-12-11-20(15-25(28)35)39-33(42)23-17-38-41(21-7-3-2-4-8-21)31(23)22-9-5-6-10-24(22)34/h2-19H,1H3,(H,39,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16055

((2S)-N-[(1S)-1-{[(1S,2S)-1-[(1R,2R)-2-{[(1S)-1-(bu...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)NCCCC)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C34H63N5O6S/c1-10-11-16-35-34(45)29(22(6)7)39-31(42)25-14-12-13-24(25)30(41)27(18-20(2)3)38-32(43)26(15-17-46-9)37-33(44)28(19-21(4)5)36-23(8)40/h20-22,24-30,41H,10-19H2,1-9H3,(H,35,45)(H,36,40)(H,37,44)(H,38,43)(H,39,42)/t24-,25-,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM16053

((2S)-2-{[(1R,2R)-2-[(1S,2S)-2-[(2S)-2-[(2S)-2-acet...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C30H54N4O7S/c1-16(2)14-23(26(36)20-10-9-11-21(20)27(37)34-25(18(5)6)30(40)41)33-28(38)22(12-13-42-8)32-29(39)24(15-17(3)4)31-19(7)35/h16-18,20-26,36H,9-15H2,1-8H3,(H,31,35)(H,32,39)(H,33,38)(H,34,37)(H,40,41)/t20-,21-,22+,23+,24+,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | 25 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177425

(CHEMBL537420 | N-(4-carbamimidoylbenzyl)-2-(3-(2,5...)Show SMILES Cc1ccc(C)c(c1)S(=O)(=O)Nc1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1O Show InChI InChI=1S/C25H28N4O4S/c1-15-4-5-17(3)22(12-15)34(32,33)29-21-11-6-16(2)20(24(21)31)13-23(30)28-14-18-7-9-19(10-8-18)25(26)27/h4-12,29,31H,13-14H2,1-3H3,(H3,26,27)(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50115202

(CHEMBL3608550)Show SMILES COc1cccc(c1)-c1c(cnn1-c1ccccc1)C(=O)Nc1ccc(Oc2ccnc3cc(sc23)-c2cn(C)cn2)c(F)c1 Show InChI InChI=1S/C34H25FN6O3S/c1-40-19-28(37-20-40)31-17-27-33(45-31)30(13-14-36-27)44-29-12-11-22(16-26(29)35)39-34(42)25-18-38-41(23-8-4-3-5-9-23)32(25)21-7-6-10-24(15-21)43-2/h3-20H,1-2H3,(H,39,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50294877
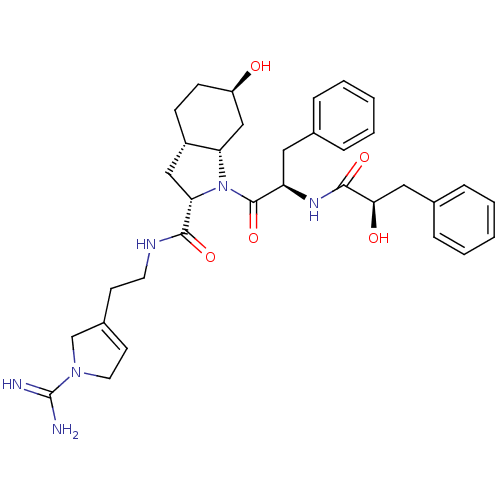
((2R,3AS,6R,7AS)-N-(2-{1-[AMINO(IMINO)METHYL]-2,5-D...)Show SMILES NC(=N)N1CC=C(CCNC(=O)[C@@H]2C[C@@H]3CC[C@@H](O)C[C@@H]3N2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](O)Cc2ccccc2)C1 |r,t:5| Show InChI InChI=1S/C34H44N6O5/c35-34(36)39-16-14-24(21-39)13-15-37-31(43)29-19-25-11-12-26(41)20-28(25)40(29)33(45)27(17-22-7-3-1-4-8-22)38-32(44)30(42)18-23-9-5-2-6-10-23/h1-10,14,25-30,41-42H,11-13,15-21H2,(H3,35,36)(H,37,43)(H,38,44)/t25-,26+,27+,28-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50114398
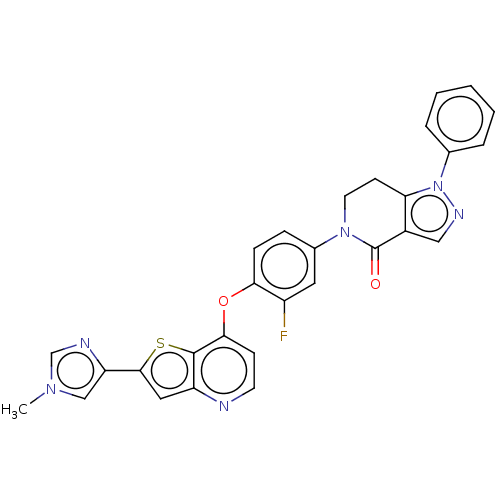
(CHEMBL3609808)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(cc3F)N3CCc4c(cnn4-c4ccccc4)C3=O)c2s1 Show InChI InChI=1S/C29H21FN6O2S/c1-34-16-23(32-17-34)27-14-22-28(39-27)26(9-11-31-22)38-25-8-7-19(13-21(25)30)35-12-10-24-20(29(35)37)15-33-36(24)18-5-3-2-4-6-18/h2-9,11,13-17H,10,12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc. / ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3706-10 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.034
BindingDB Entry DOI: 10.7270/Q2SJ1NDW |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50317323

(CHEMBL1094718 | N-(3-fluoro-4-(2-(1-methyl-1H-imid...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-14-21(34-15-37)24-12-20-25(41-24)23(9-10-33-20)40-22-8-7-16(11-19(22)29)36-27(39)18-13-35-38(26(18)28(30,31)32)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON phosphorylation in human PC3 cells |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50317323

(CHEMBL1094718 | N-(3-fluoro-4-(2-(1-methyl-1H-imid...)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cnn(c4C(F)(F)F)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C28H18F4N6O2S/c1-37-14-21(34-15-37)24-12-20-25(41-24)23(9-10-33-20)40-22-8-7-16(11-19(22)29)36-27(39)18-13-35-38(26(18)28(30,31)32)17-5-3-2-4-6-17/h2-15H,1H3,(H,36,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ChemRF Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RON phosphorylation in human HT29 cells |
Bioorg Med Chem Lett 25: 2527-31 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.056
BindingDB Entry DOI: 10.7270/Q21C1ZMR |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50115200
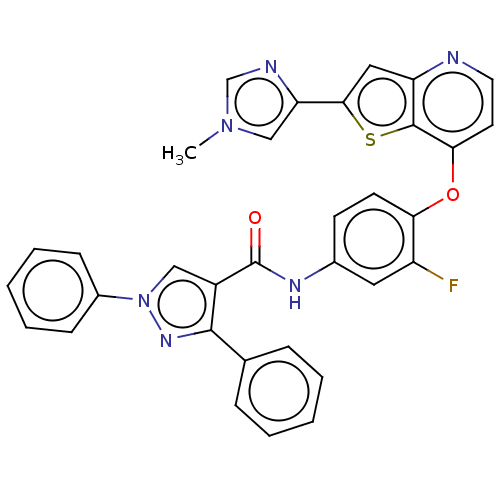
(CHEMBL3608545)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(NC(=O)c4cn(nc4-c4ccccc4)-c4ccccc4)cc3F)c2s1 Show InChI InChI=1S/C33H23FN6O2S/c1-39-19-27(36-20-39)30-17-26-32(43-30)29(14-15-35-26)42-28-13-12-22(16-25(28)34)37-33(41)24-18-40(23-10-6-3-7-11-23)38-31(24)21-8-4-2-5-9-21/h2-20H,1H3,(H,37,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc./ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3810-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.080
BindingDB Entry DOI: 10.7270/Q2028T99 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50114396

(CHEMBL3609807)Show SMILES Cn1cnc(c1)-c1cc2nccc(Oc3ccc(cc3F)N3CCCc4c(cnn4-c4ccccc4)C3=O)c2s1 Show InChI InChI=1S/C30H23FN6O2S/c1-35-17-24(33-18-35)28-15-23-29(40-28)27(11-12-32-23)39-26-10-9-20(14-22(26)31)36-13-5-8-25-21(30(36)38)16-34-37(25)19-6-3-2-4-7-19/h2-4,6-7,9-12,14-18H,5,8,13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires ChemRF Inc. / ChemRF Laboratories Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human RON |
Bioorg Med Chem Lett 25: 3706-10 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.034
BindingDB Entry DOI: 10.7270/Q2SJ1NDW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177421

(CHEMBL558235 | N-(4-carbamimidoylbenzyl)-2-(3-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)Nc1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1O Show InChI InChI=1S/C25H28N4O6S/c1-15-4-10-20(29-36(32,33)18-9-11-21(34-2)22(12-18)35-3)24(31)19(15)13-23(30)28-14-16-5-7-17(8-6-16)25(26)27/h4-12,29,31H,13-14H2,1-3H3,(H3,26,27)(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1 [1-453]
(Homo sapiens (Human)) | BDBM16051

((4S)-4-amino-4-{[(1S)-1-{[(1S)-2-carbamoyl-1-{[(1S...)Show SMILES [H][C@]1(CCC[C@H]1C(=O)N[C@@H](C)C(O)=O)[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C |r| Show InChI InChI=1S/C29H50N6O10/c1-13(2)11-19(24(39)16-7-6-8-17(16)25(40)32-15(5)29(44)45)33-27(42)20(12-21(31)36)34-28(43)23(14(3)4)35-26(41)18(30)9-10-22(37)38/h13-20,23-24,39H,6-12,30H2,1-5H3,(H2,31,36)(H,32,40)(H,33,42)(H,34,43)(H,35,41)(H,37,38)(H,44,45)/t15-,16+,17+,18-,19-,20-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Universite de Montreal at Succursale Centre-Ville
| Assay Description
Inhibition assays were done in black 96-well plates by adding test compounds to the respective enzyme in assay buffer. Plates were incubated at room ... |
J Med Chem 48: 5175-90 (2005)
Article DOI: 10.1021/jm050142+
BindingDB Entry DOI: 10.7270/Q2WM1BPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data