

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336739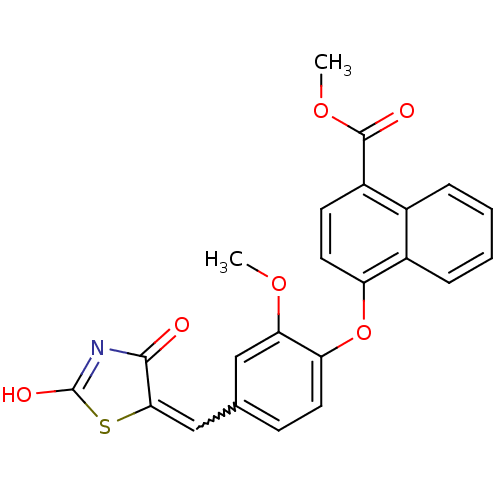 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336759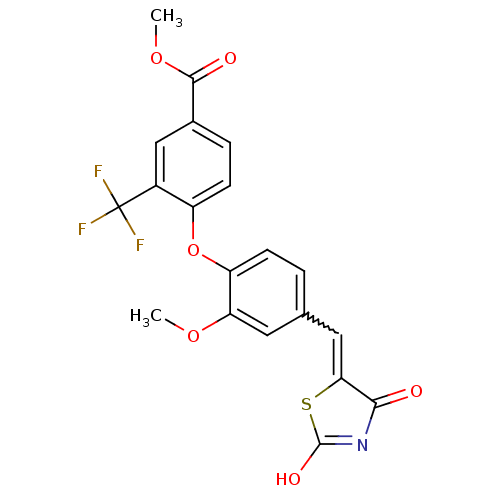 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336760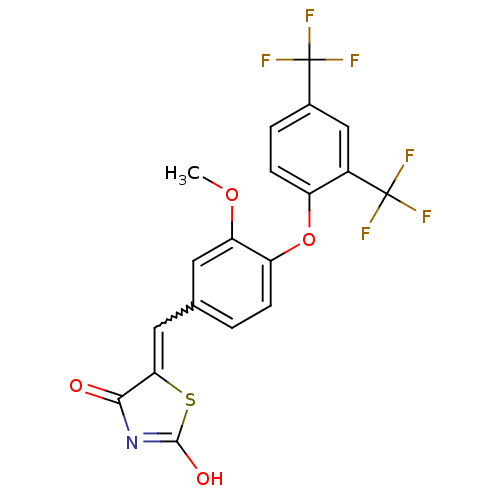 (5-[4-(2,4-Bis-trifluoromethylphenoxy)-3-methoxyben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336753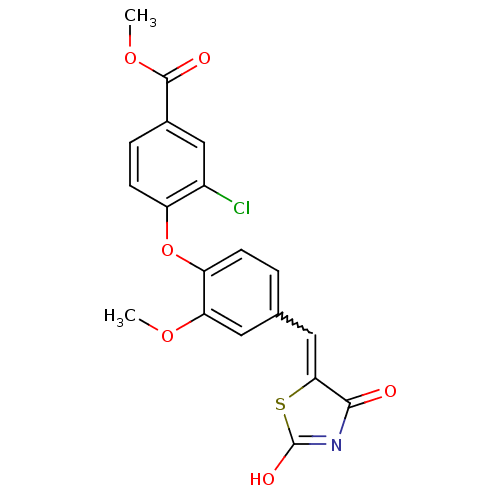 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336731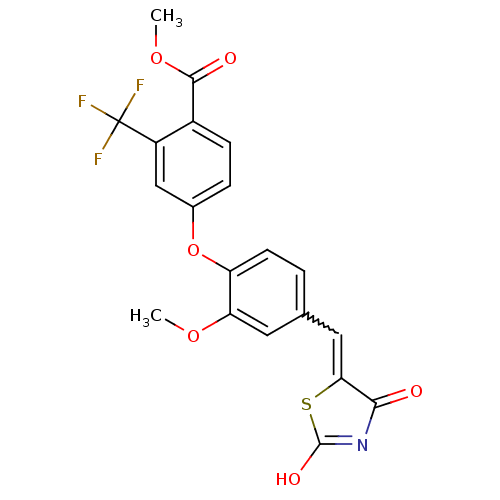 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336738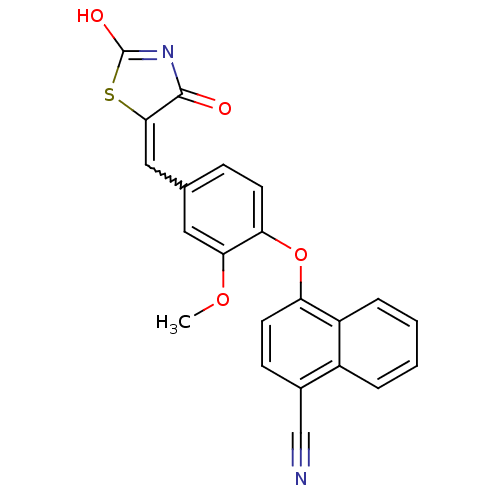 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336761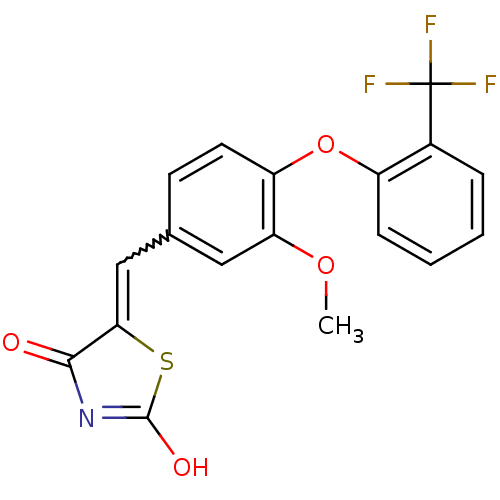 (5-[4-(2-Trifluoromethylphenoxy)-3-methoxybenzylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336762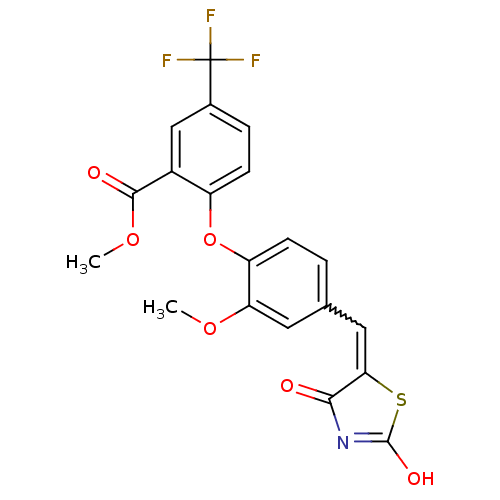 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336756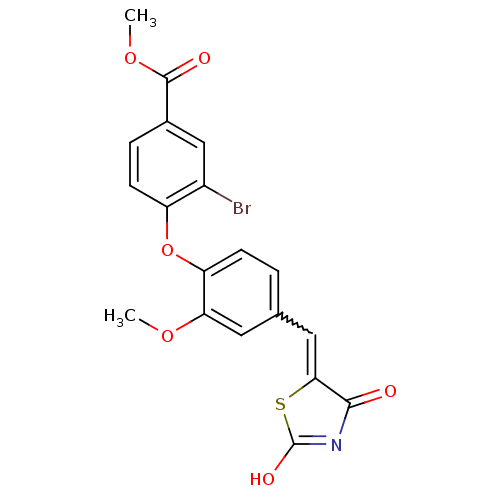 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336739 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336757 (5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336754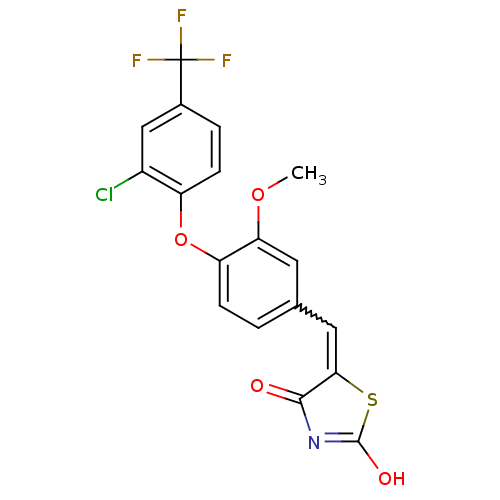 (5-[4-(2-Chloro-4-trifluoromethylphenoxy)-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM22420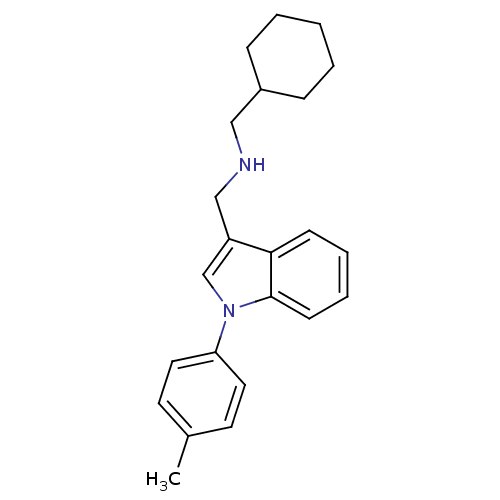 ((cyclohexylmethyl)({[1-(4-methylphenyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336735 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336752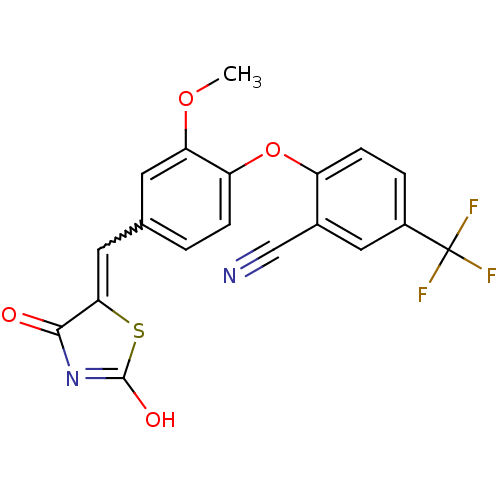 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336747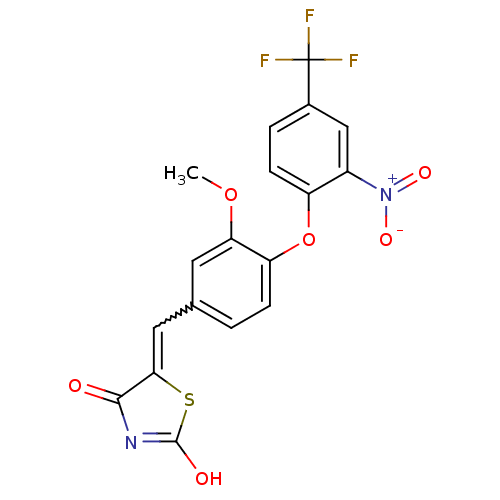 (5-[3-Methoxy-4-(2-nitro-4-trifluoromethylphenoxy)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336751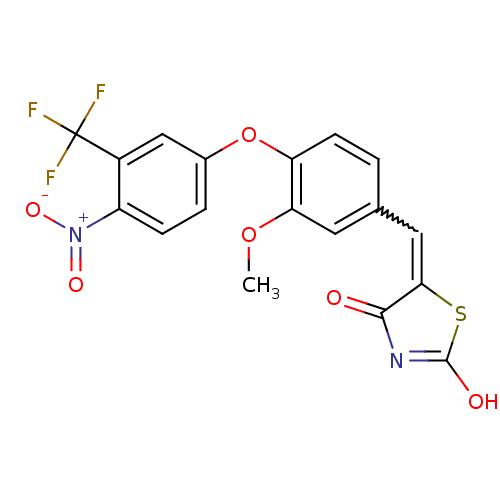 (5-[3-Methoxy-4-(4-nitro-3-trifluoromethylphenoxy)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336748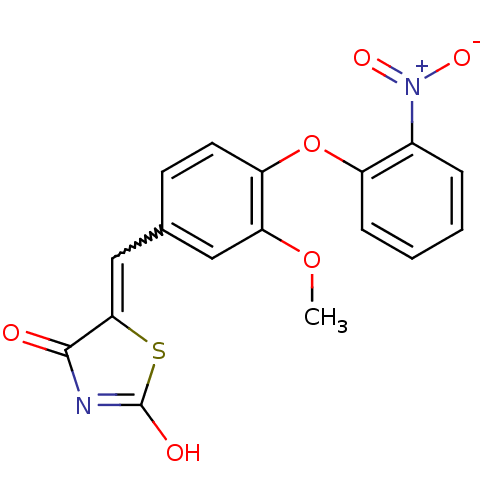 (5-[3-Methoxy-4-(2-nitrophenoxy)benzylidene]thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336743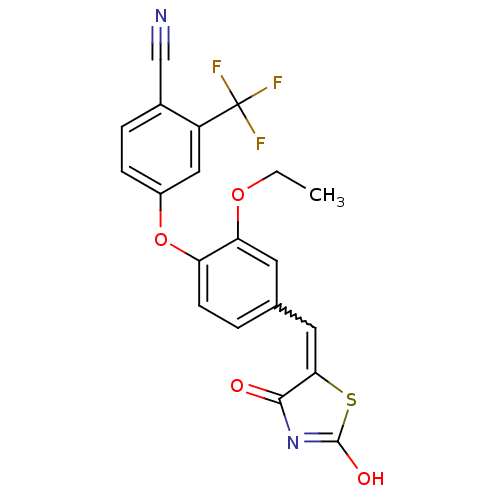 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-etho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50155833 (3-[4-(2,4-Bis-trifluoromethyl-benzyloxy)-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336749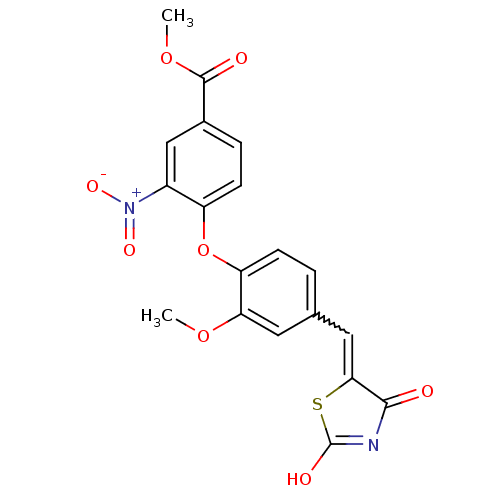 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336732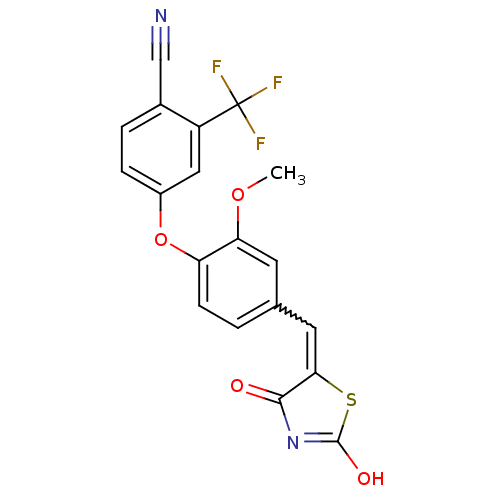 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336766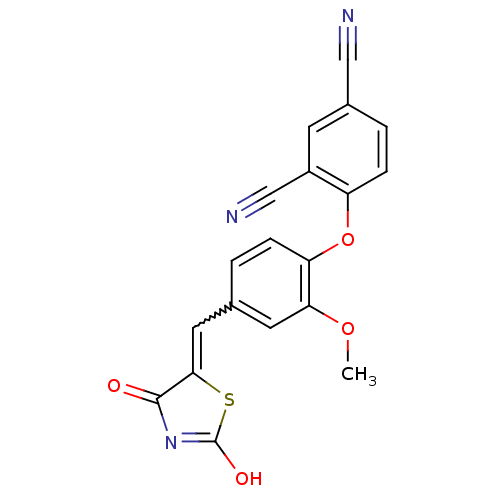 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336755 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to ERbeta by fluorescence polarization assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human ERRalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336734 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336761 (5-[4-(2-Trifluoromethylphenoxy)-3-methoxybenzylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336741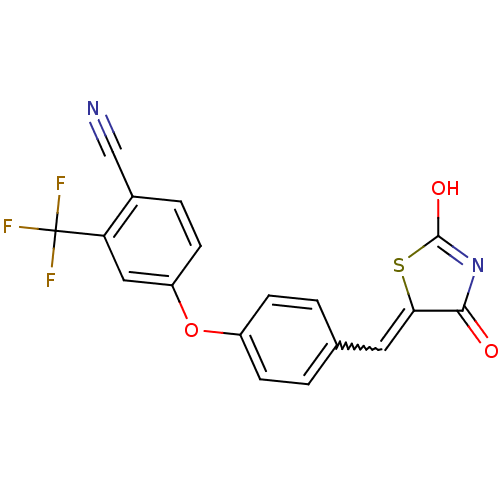 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336758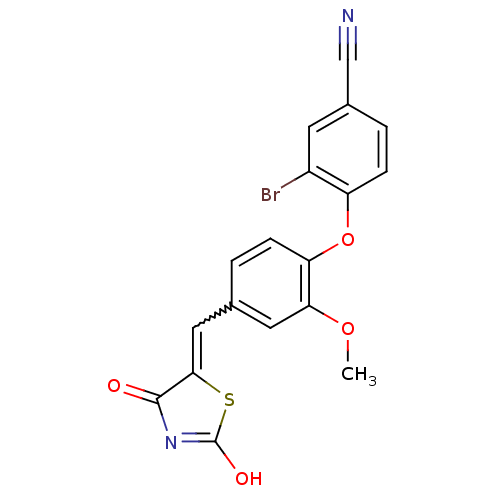 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336744 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336739 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336742 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336733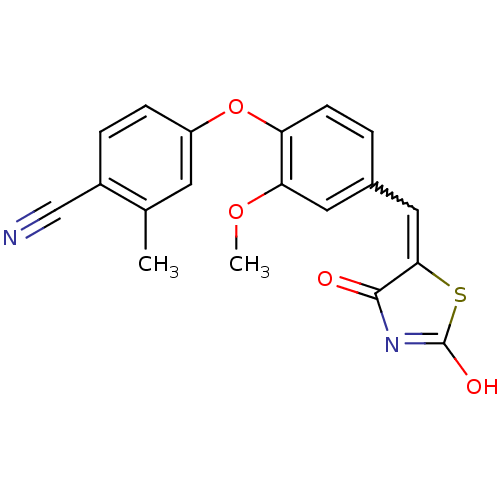 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336737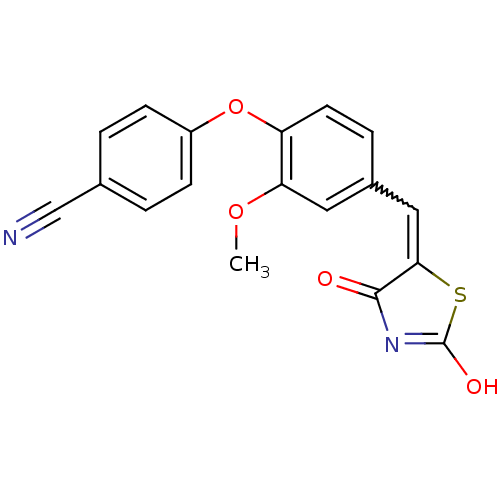 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336764 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336750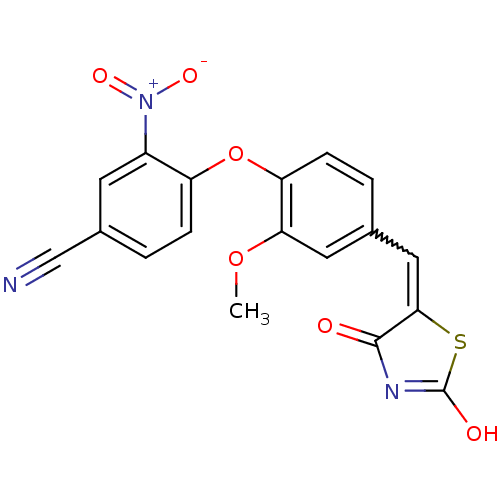 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336740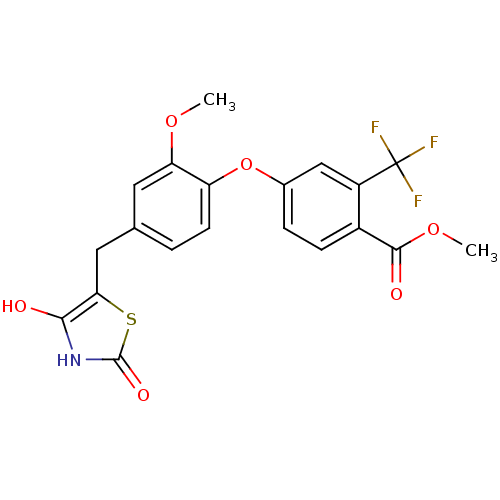 (4-[4-(2,4-Dioxothiazolidin-5-ylmethyl)-2-methoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336736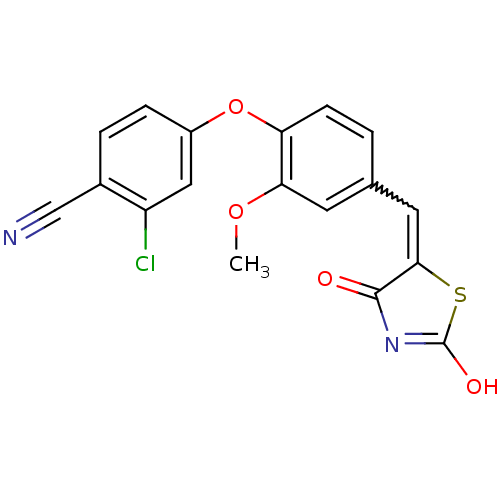 (2-Chloro-4-[4-(2,4-dioxothiazolidin-5-ylidenemethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336759 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336745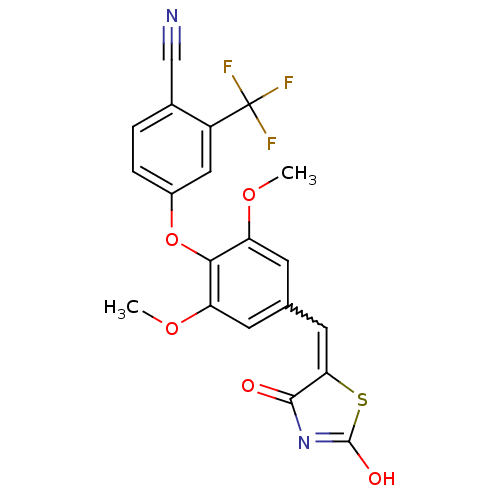 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2,6-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336761 (5-[4-(2-Trifluoromethylphenoxy)-3-methoxybenzylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to ERalpha by fluorescence polarization assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336754 (5-[4-(2-Chloro-4-trifluoromethylphenoxy)-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336738 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336765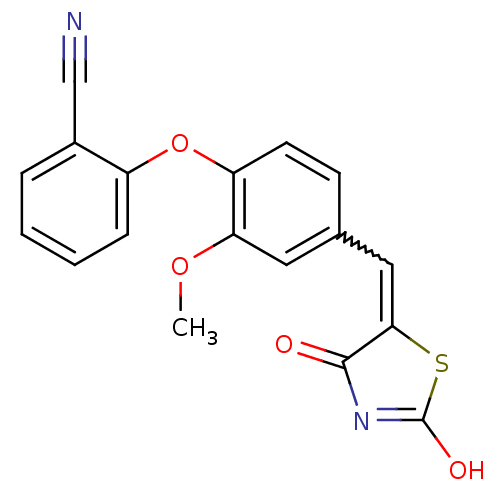 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336753 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336746 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336757 (5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRgamma LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336756 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336747 (5-[3-Methoxy-4-(2-nitro-4-trifluoromethylphenoxy)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336757 (5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336738 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336752 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336751 (5-[3-Methoxy-4-(4-nitro-3-trifluoromethylphenoxy)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336753 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336758 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERbeta by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336735 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at LXRbeta by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at LXRalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at RARalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336762 (2-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336759 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma by fluorescence polarization assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336759 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336761 (5-[4-(2-Trifluoromethylphenoxy)-3-methoxybenzylide...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336738 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336757 (5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxyb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336748 (5-[3-Methoxy-4-(2-nitrophenoxy)benzylidene]thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336750 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336739 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336731 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336760 (5-[4-(2,4-Bis-trifluoromethylphenoxy)-3-methoxyben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336754 (5-[4-(2-Chloro-4-trifluoromethylphenoxy)-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336755 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336753 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336733 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336732 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336754 (5-[4-(2-Chloro-4-trifluoromethylphenoxy)-3-methoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336749 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARdelta LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human ERalpha LBD in human MCF7 cells assessed as inhibition of estradiol-induced cell proliferation after up to 6 days by cel... | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity to PPARgamma by fluorescence polarization assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen-related receptor gamma (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human ERRgamma LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human ERbeta LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human RXRalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50127222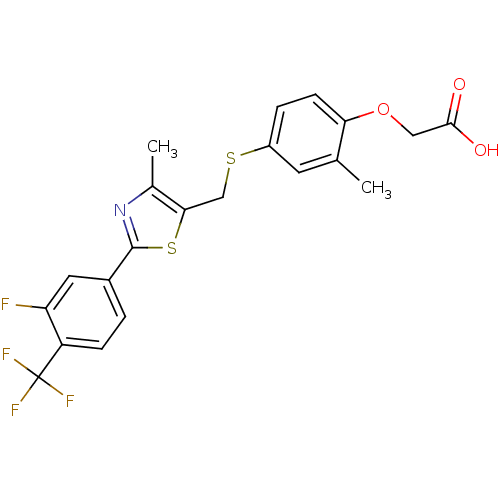 (CHEMBL38508 | GW-0742 | {4-[2-(3-Fluoro-4-trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARdelta LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoform 1 of Steroid hormone receptor ERR2 (ERRbeta2-delta10) (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ZFP-fused ERRbeta LBD expressed in HEK293 cells by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at RARalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human ERbeta LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292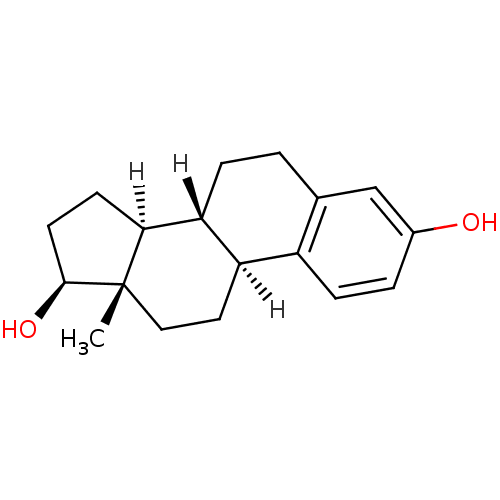 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human ERbeta LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681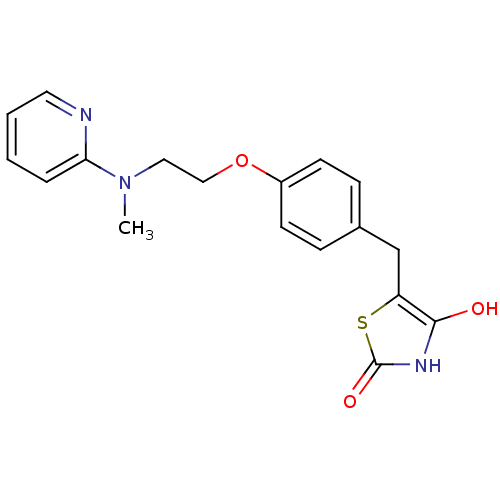 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARgamma LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human RXRalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50099491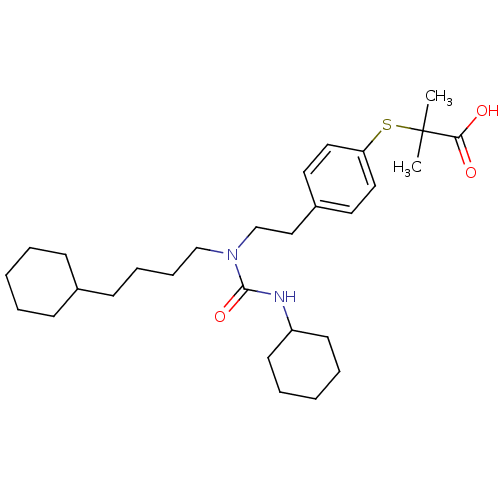 (2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at LXRbeta by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >8.30E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at LXRalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993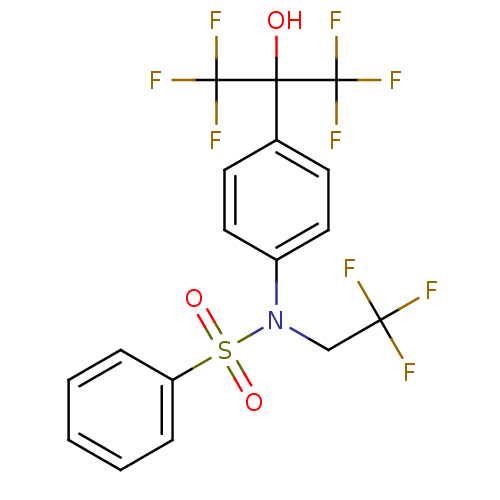 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at LXRalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50052414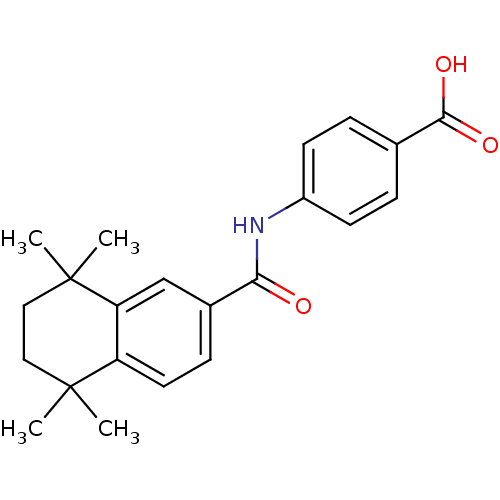 (4-(1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthale...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at RARalpha by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human RXRalpha LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at LXRbeta by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human PPARdelta LBD by cell based luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50336730 (4-[4-(2,4-Dioxothiazolidin-5-ylidenemethyl)-2-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human ERalpha LBD in human MCF7 cells assessed as induction of cell proliferation after up to 6 days by celltiter-glo assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50336763 (4-(5-(2,5-dimethylphenyl)-1-methyl-1H-pyrazol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at 6his-tagged ERRalpha LBD assessed as recruitment of GST-labeled coactivator Scr2 by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Steroid hormone receptor ERR2 (ERRbeta2-delta10) (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ZFP-fused ERRbeta LBD expressed in HEK293 cells by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at human ERalpha LBD in human MCF7 cells assessed as induction of cell proliferation after up to 6 days by celltiter-glo assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||