

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50433561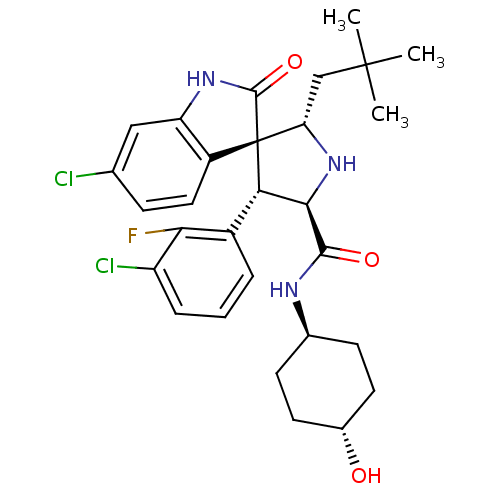 (CHEMBL2381408) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant human His6-tagged HDM2 (1 to 118 residues) assessed as reduction in PMDM6-F binding incubated for 15 to 30 mins by fl... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01524 BindingDB Entry DOI: 10.7270/Q2Z03D1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50444037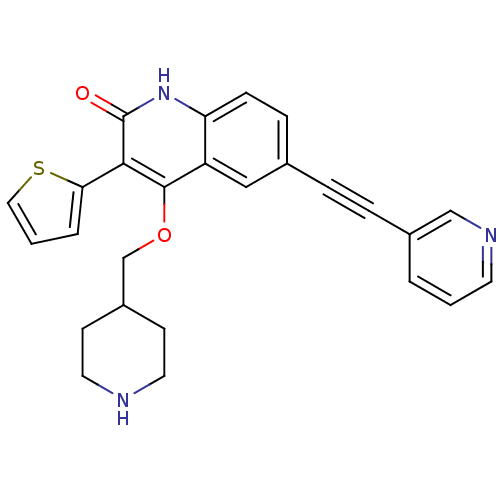 (CHEMBL3092564 | US8680116, 1-15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119685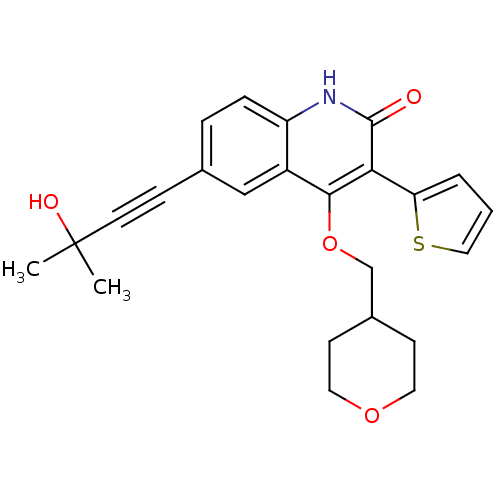 (US8680116, 1-57) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119686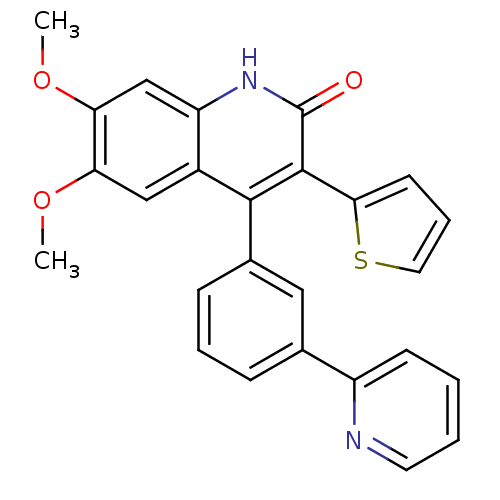 (US8680116, 1-63) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119682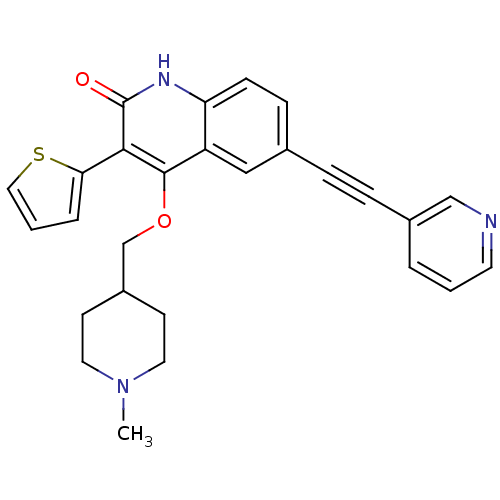 (US8680116, 1-23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119680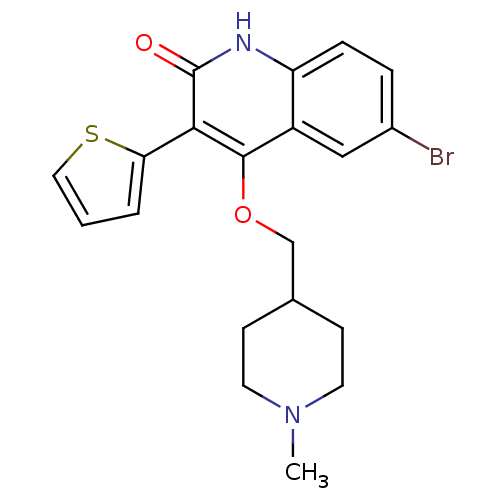 (US8680116, 1-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119687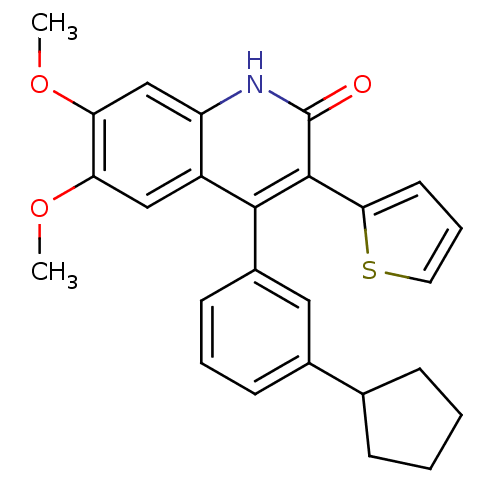 (US8680116, 1-85) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119683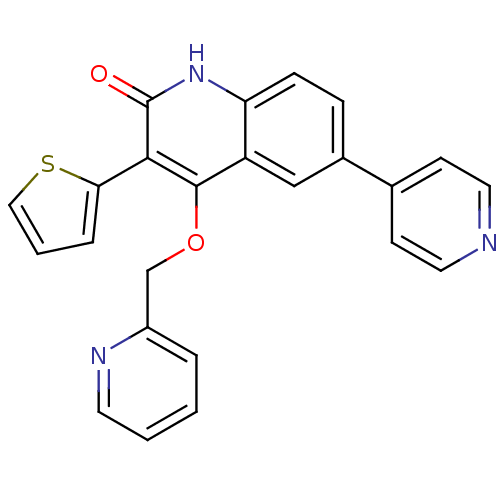 (US8680116, 1-48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119681 (US8680116, 1-18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50460673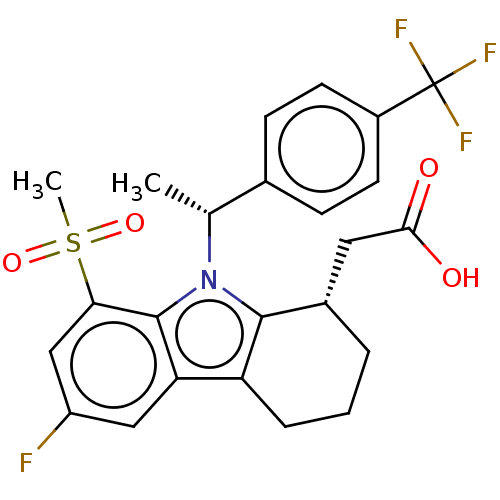 (CHEMBL4229054) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc. Curated by ChEMBL | Assay Description Antagonist activity at DP1 receptor (unknown origin) | Bioorg Med Chem Lett 28: 1122-1126 (2018) Article DOI: 10.1016/j.bmcl.2018.01.039 BindingDB Entry DOI: 10.7270/Q2QV3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50460670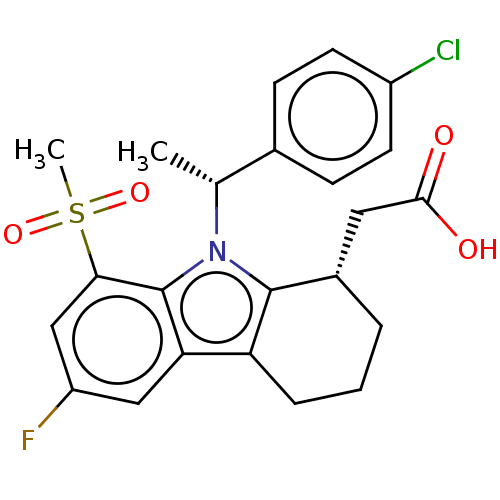 (CHEMBL4228792) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc. Curated by ChEMBL | Assay Description Antagonist activity at DP1 receptor (unknown origin) | Bioorg Med Chem Lett 28: 1122-1126 (2018) Article DOI: 10.1016/j.bmcl.2018.01.039 BindingDB Entry DOI: 10.7270/Q2QV3Q4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM119684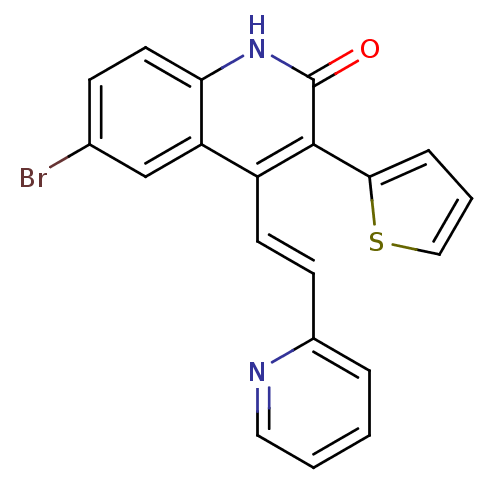 (US8680116, 1-51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluorescence polarization... | US Patent US8680116 (2014) BindingDB Entry DOI: 10.7270/Q2N29VM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227653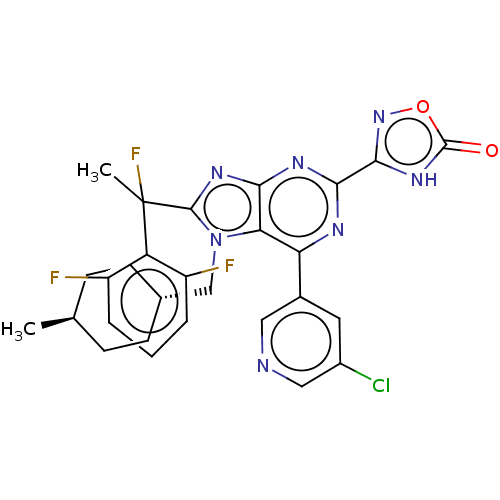 (3-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.156 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227651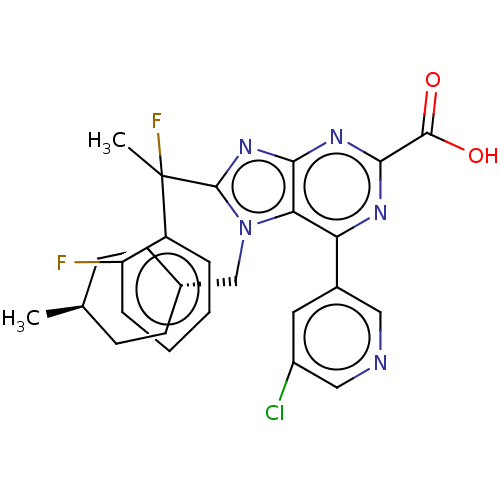 (6-(5-chloropyridin-3-yl)- 8-[1-fluoro-1-(2- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.176 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223046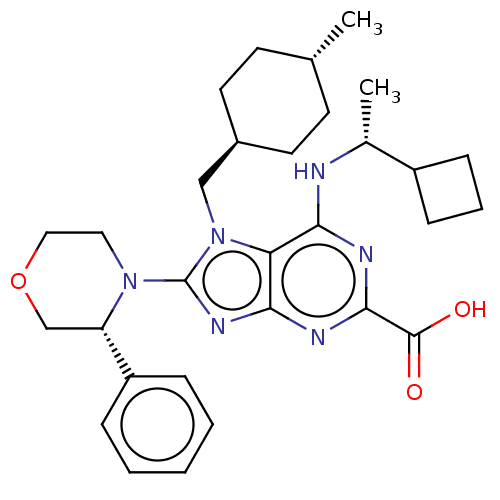 (6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.202 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224125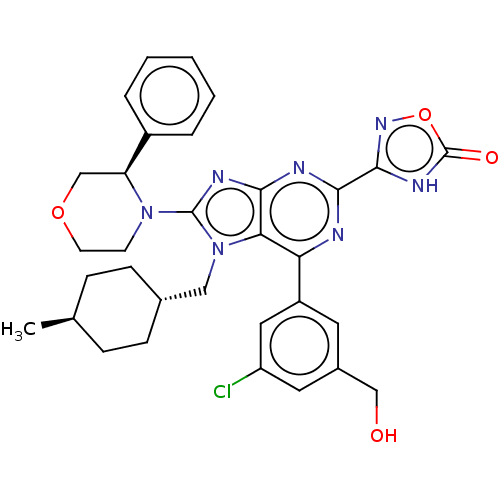 (3-{6-[3-chloro-5- (hydroxymethyl)phen- yl]-7-[(tra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.205 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515298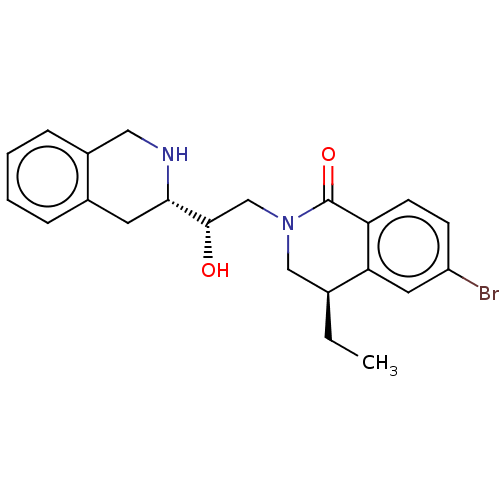 (US11098059, Example 2 | US11098059, Example 6) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224261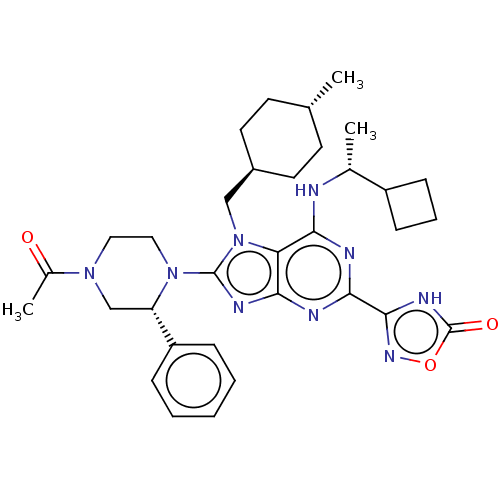 (3-{8-[(2r)-4-acetyl- 2-phenylpiperazin-1- yl]-6-{[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.215 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515310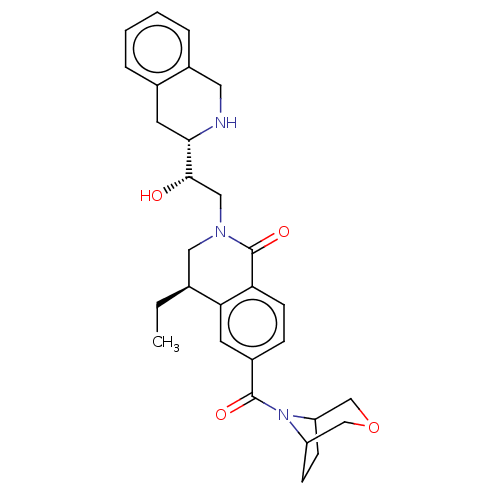 (US11098059, Example 14) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227665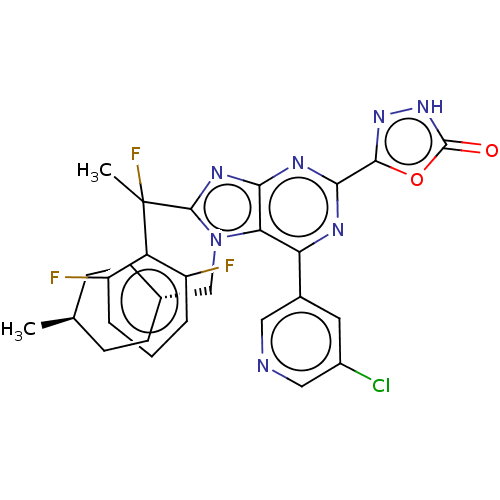 (5-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.289 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515307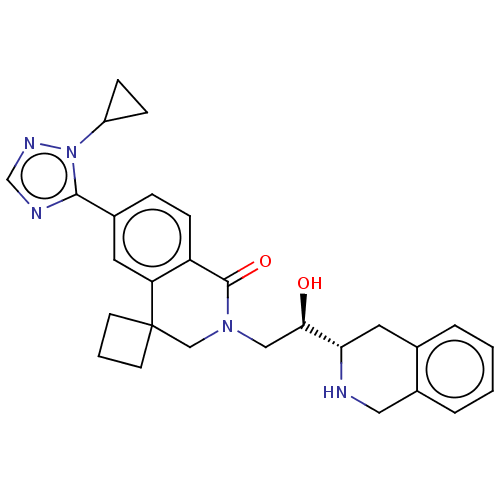 (6'-(1-cyclopropyl-1H-1,2,4- triazol-5-yl)-2'-{(2R)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515299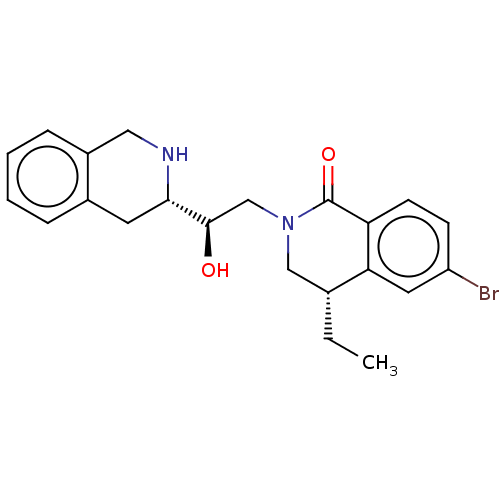 (US11098059, Example 3 | US11098059, Example 7) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227683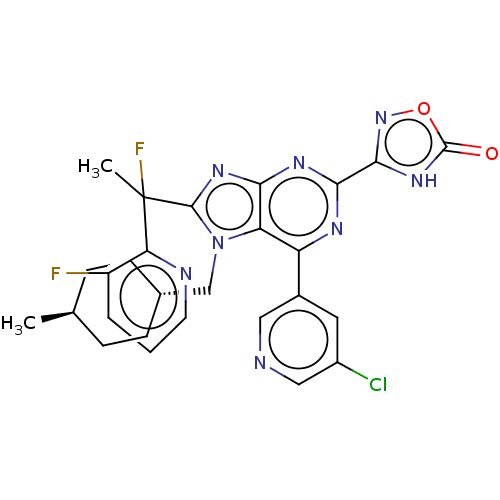 (3-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(3- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.315 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223705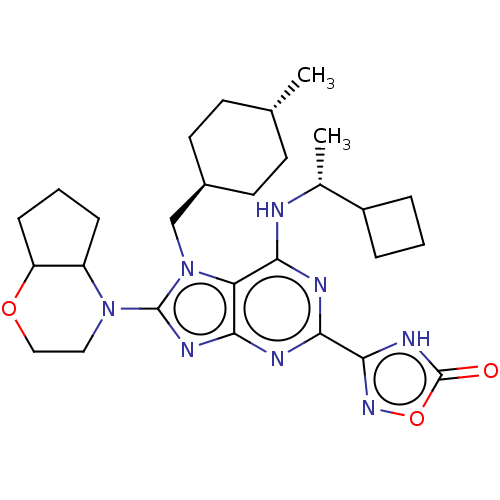 (3-(6-{[(1r)-1- cyclobutylethyl]amino}- 8- (hexahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.344 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515323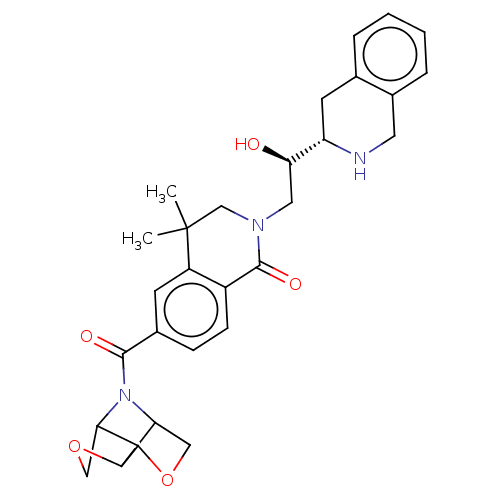 (6-(hexahydro-3,6- epiminofuro[3,2-b]furan- 7-carbo...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227669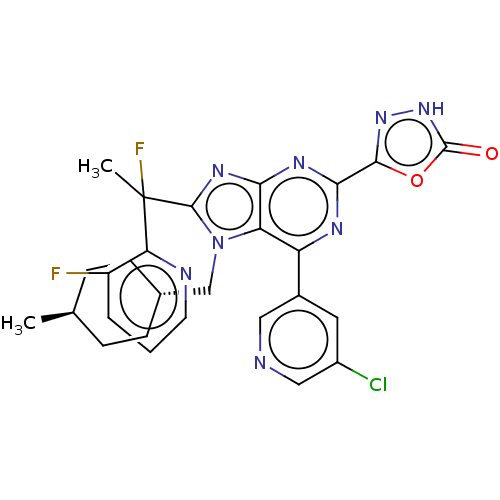 (5-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(3- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.359 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515309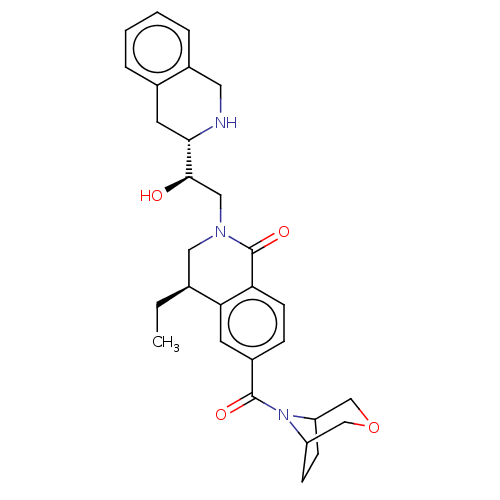 (US11098059, Example 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM223402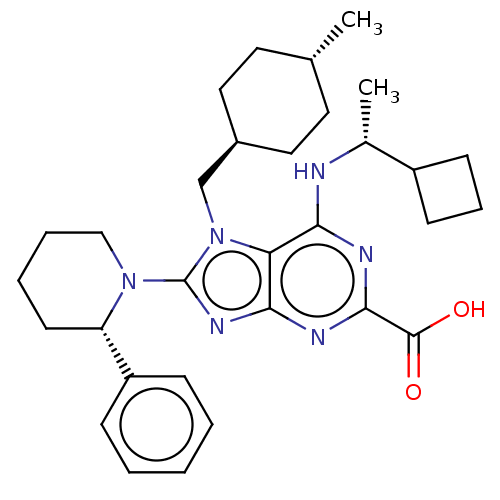 (6-{[(1r)-1- cyclobutylethyl]amino}- 7-[(trans-4- m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515306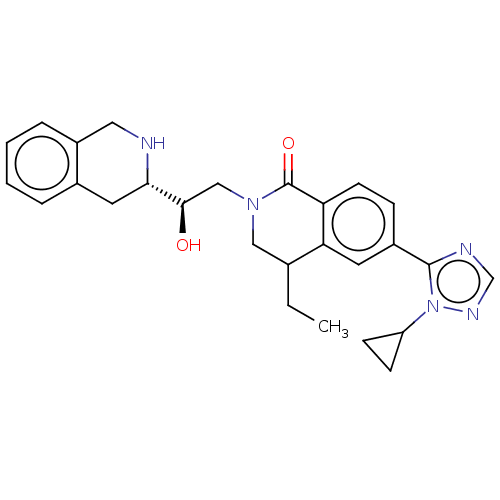 (6-(1-cyclopropyl-1H-1,2,4- triazol-5-yl)-4-ethyl-2...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM226428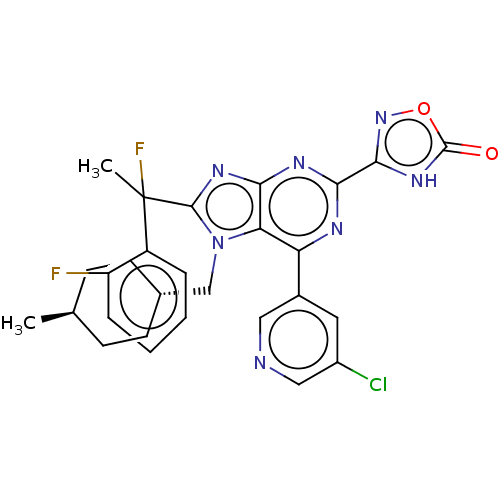 (3-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(2- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.424 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227696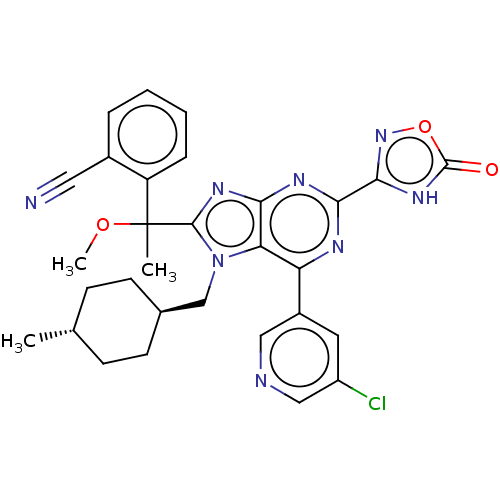 (2-{1-[6-(5-chloropyridin- 3-yl)-7-[(trans-4- methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.435 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515321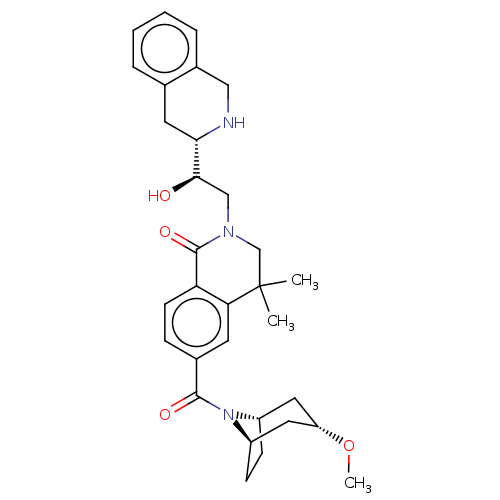 (2-((R)-2-hydroxy-2-((S)- 1,2,3,4- tetrahydroisoqui...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM227678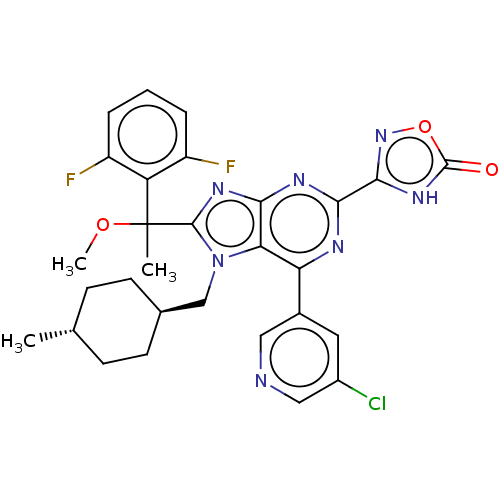 (3-{6-(5-chloropyridin-3- yl)-8-[1-(2,6- difluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.452 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM226426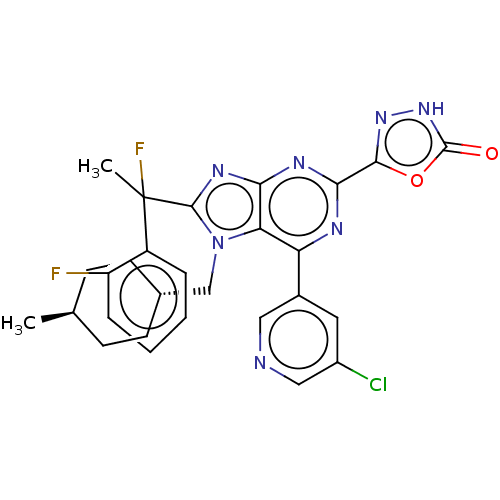 (5-{6-(5-chloropyridin-3- yl)-8-[1-fluoro-1-(2- flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.453 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methylosome protein 50/Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM515305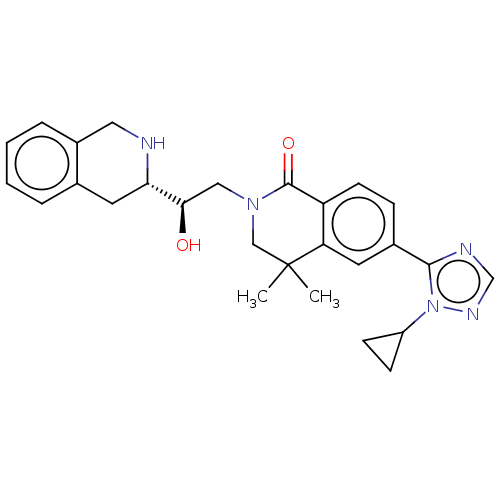 (6-(1-cyclopropyl-1H-1,2,4-triazol-5-yl)-2-{(2R)-2-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In this assay, the potency (IC50) of each compound was determined from a twenty-point (1:2 serial dilution; top compound concentration of 100000 nM) ... | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5X5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM224176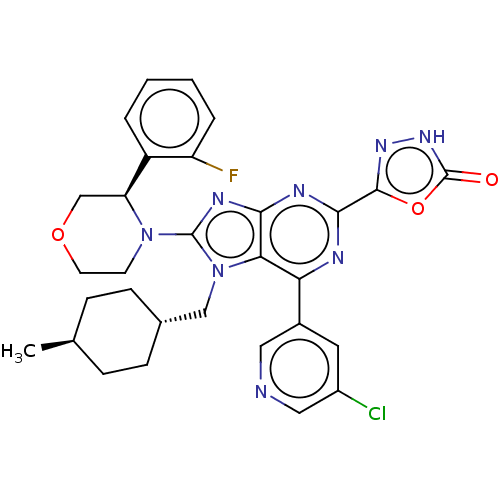 (5-{6-(5- chloropyridin-3-yl)- 8-[(3r)-3-(2- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.494 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM2 with re... | US Patent US9540377 (2017) BindingDB Entry DOI: 10.7270/Q2RF5X1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127952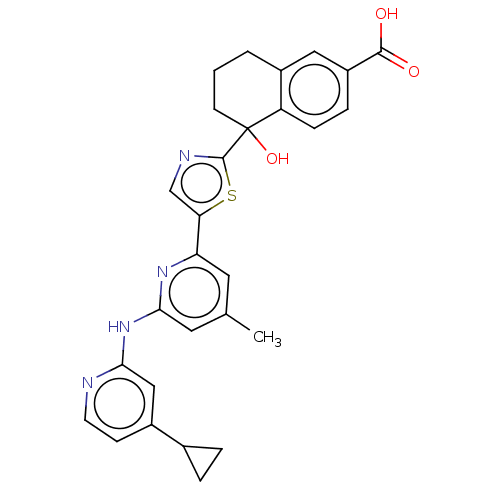 (US8796310, 1U) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM128020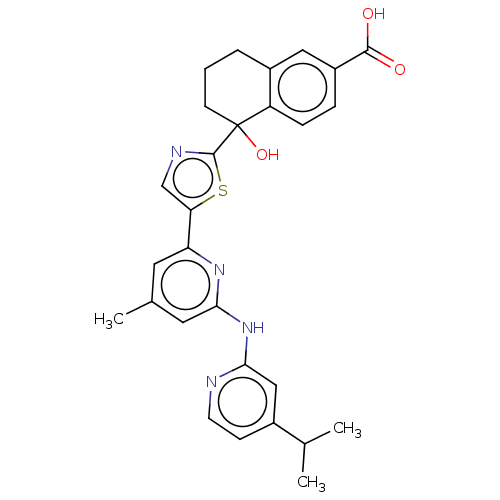 (US8796310, 1V) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127953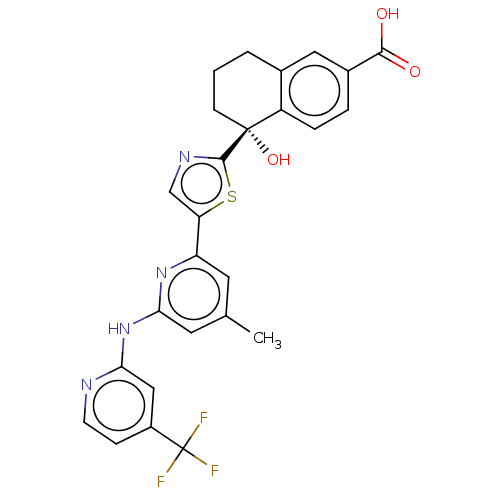 (US8796310, 2 | US8796310, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127956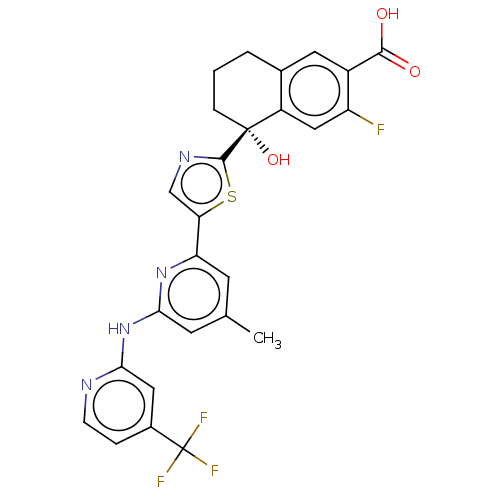 (US8796310, 4 | US8796310, 4A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127956 (US8796310, 4 | US8796310, 4A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127960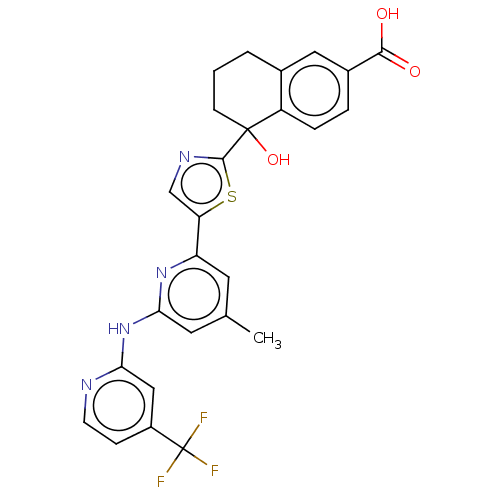 (US8796310, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127964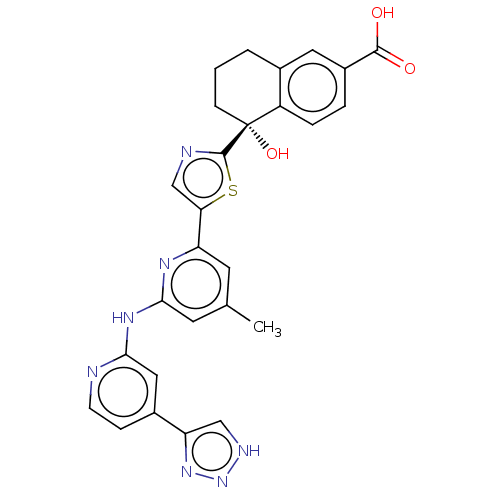 (US8796310, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127965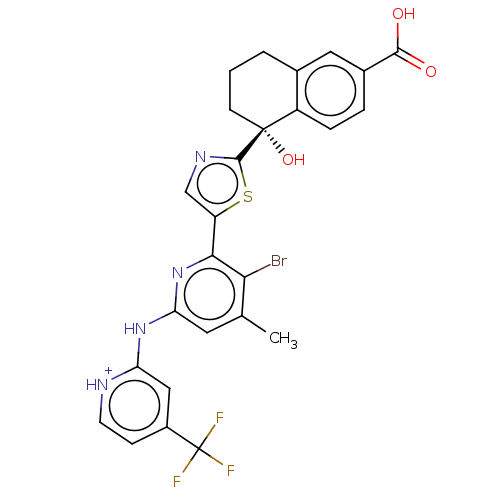 (US8796310, 12A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127969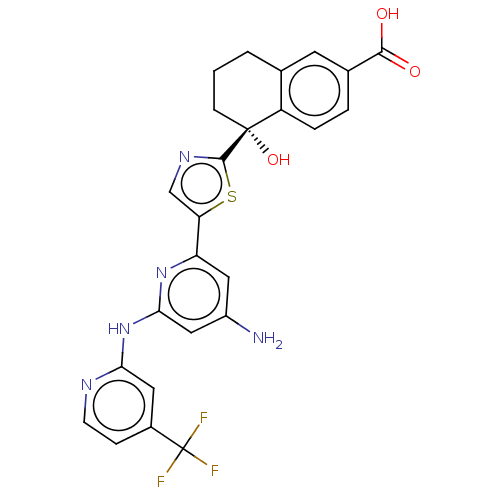 (US8796310, 13B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127972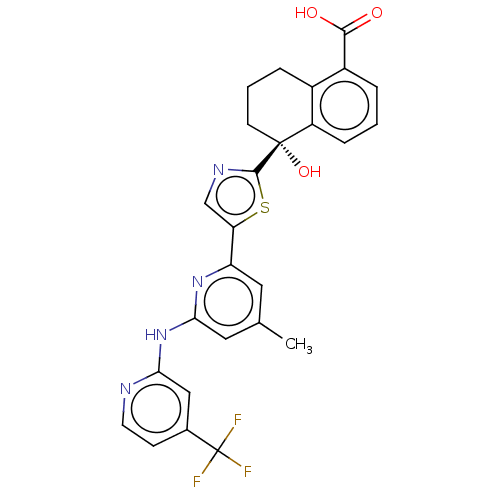 (US8796310, 14C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127973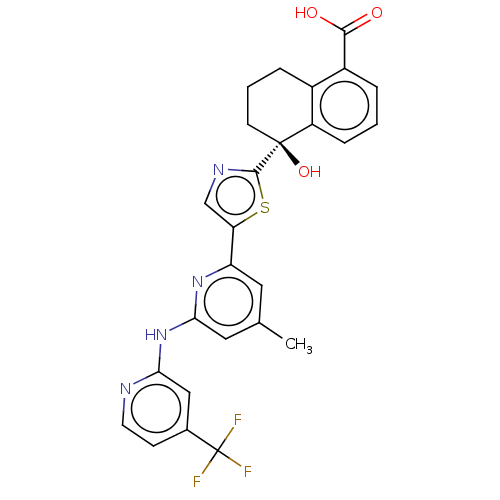 (US8796310, 14D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127975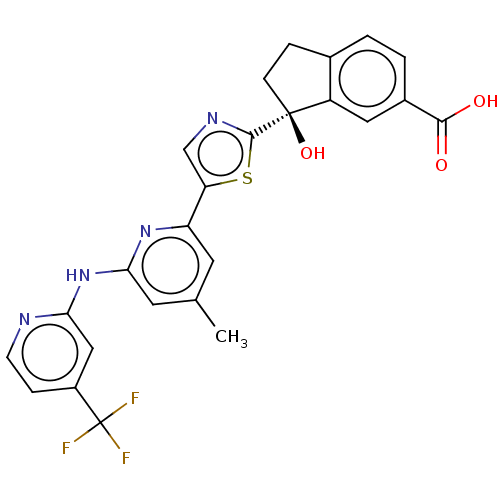 (US8796310, 15B | US8796310, 15J) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127978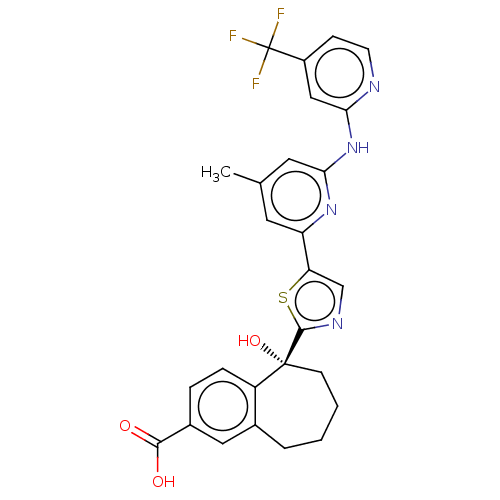 (US8796310, 15E) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM127980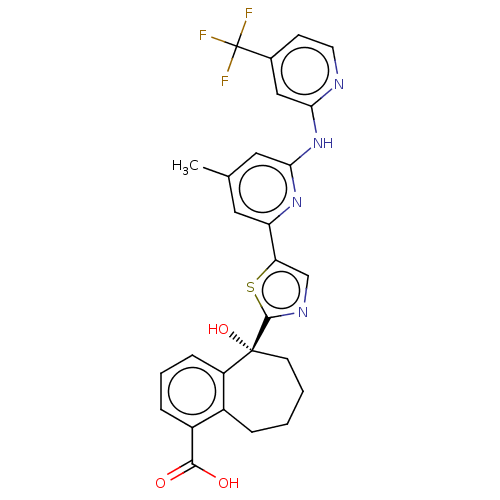 (US8796310, 15G) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A recombinant GST-hSyk fusion protein was used to measure potency of compounds to inhibit human Syk activity. The recombinant human GST-Syk (Cama Bio... | US Patent US8796310 (2014) BindingDB Entry DOI: 10.7270/Q29W0D61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3216 total ) | Next | Last >> |