 Found 108 hits of Enzyme Inhibition Constant Data
Found 108 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119704
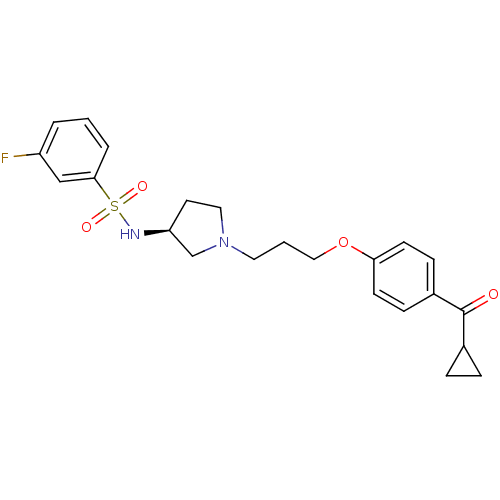
(CHEMBL104809 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-19-3-1-4-22(15-19)31(28,29)25-20-11-13-26(16-20)12-2-14-30-21-9-7-18(8-10-21)23(27)17-5-6-17/h1,3-4,7-10,15,17,20,25H,2,5-6,11-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119707
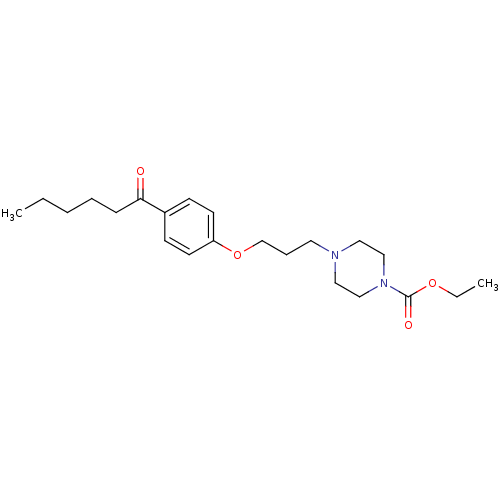
(4-[3-(4-Hexanoyl-phenoxy)-propyl]-piperazine-1-car...)Show InChI InChI=1S/C22H34N2O4/c1-3-5-6-8-21(25)19-9-11-20(12-10-19)28-18-7-13-23-14-16-24(17-15-23)22(26)27-4-2/h9-12H,3-8,13-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards rats Histamine type 3 (H3) receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119710
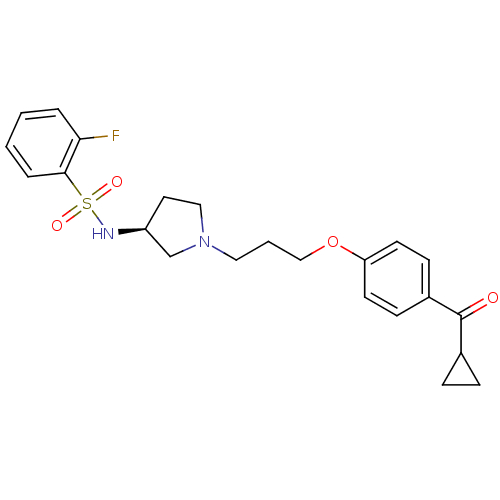
(CHEMBL104618 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-21-4-1-2-5-22(21)31(28,29)25-19-12-14-26(16-19)13-3-15-30-20-10-8-18(9-11-20)23(27)17-6-7-17/h1-2,4-5,8-11,17,19,25H,3,6-7,12-16H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119721
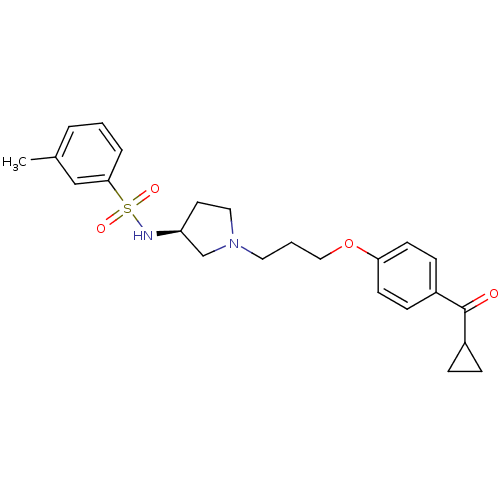
(CHEMBL103027 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-4-2-5-23(16-18)31(28,29)25-21-12-14-26(17-21)13-3-15-30-22-10-8-20(9-11-22)24(27)19-6-7-19/h2,4-5,8-11,16,19,21,25H,3,6-7,12-15,17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119735
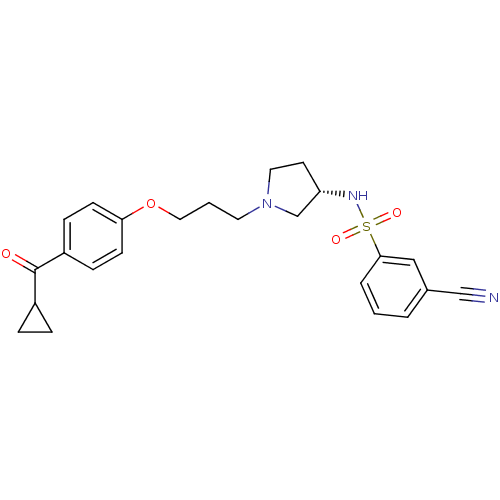
(3-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2cccc(c2)C#N)cc1 Show InChI InChI=1S/C24H27N3O4S/c25-16-18-3-1-4-23(15-18)32(29,30)26-21-11-13-27(17-21)12-2-14-31-22-9-7-20(8-10-22)24(28)19-5-6-19/h1,3-4,7-10,15,19,21,26H,2,5-6,11-14,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119722
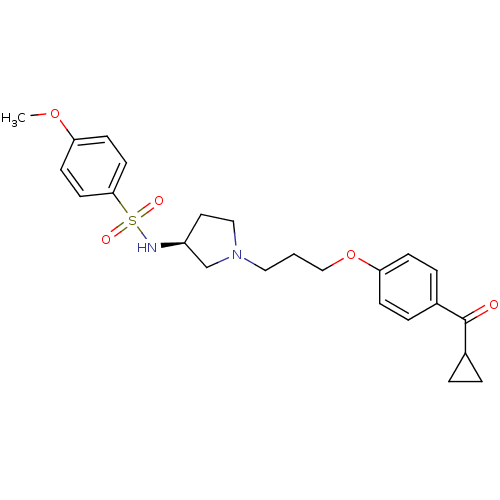
(CHEMBL107162 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O5S/c1-30-21-9-11-23(12-10-21)32(28,29)25-20-13-15-26(17-20)14-2-16-31-22-7-5-19(6-8-22)24(27)18-3-4-18/h5-12,18,20,25H,2-4,13-17H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119705
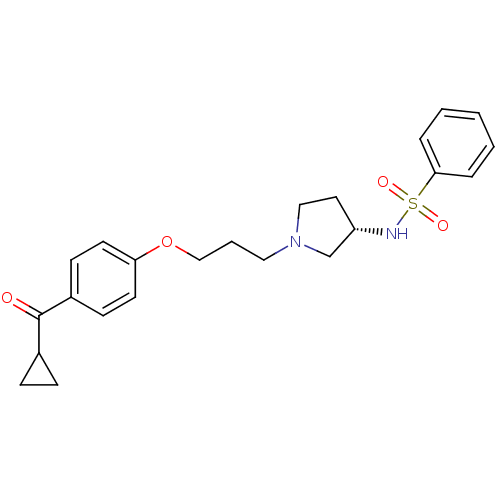
(CHEMBL101691 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N2O4S/c26-23(18-7-8-18)19-9-11-21(12-10-19)29-16-4-14-25-15-13-20(17-25)24-30(27,28)22-5-2-1-3-6-22/h1-3,5-6,9-12,18,20,24H,4,7-8,13-17H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119712
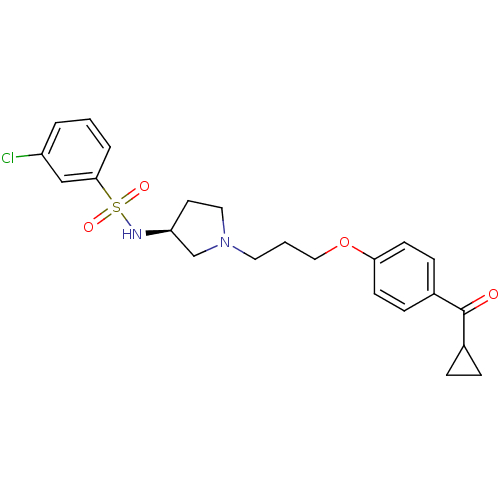
(3-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-19-3-1-4-22(15-19)31(28,29)25-20-11-13-26(16-20)12-2-14-30-21-9-7-18(8-10-21)23(27)17-5-6-17/h1,3-4,7-10,15,17,20,25H,2,5-6,11-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119701
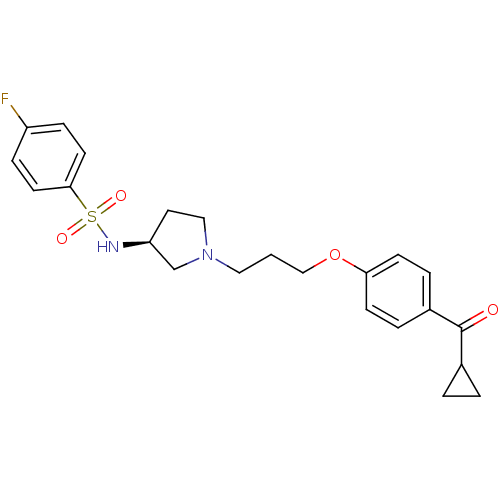
(CHEMBL104808 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119711
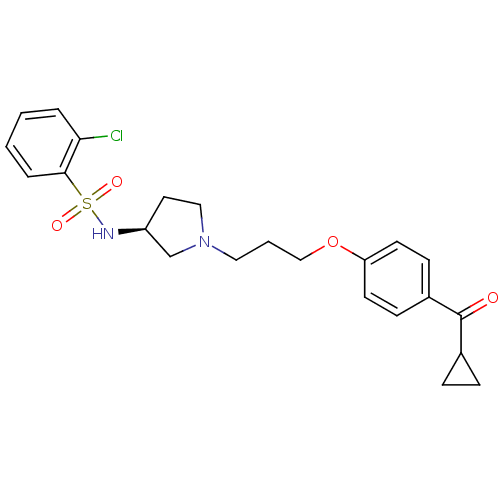
(2-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-21-4-1-2-5-22(21)31(28,29)25-19-12-14-26(16-19)13-3-15-30-20-10-8-18(9-11-20)23(27)17-6-7-17/h1-2,4-5,8-11,17,19,25H,3,6-7,12-16H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119728
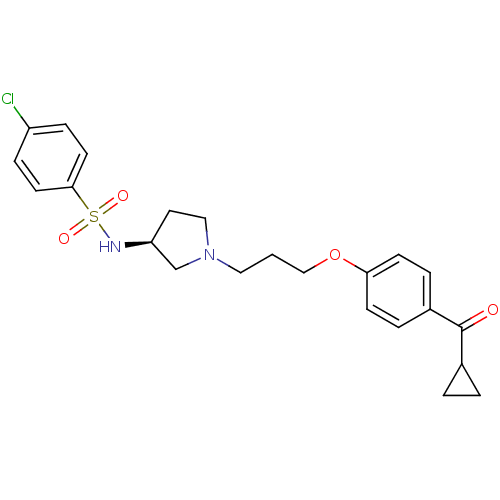
(4-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50404001

(CHEMBL2112451)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C24H27N3O4S/c25-16-18-2-10-23(11-3-18)32(29,30)26-21-12-14-27(17-21)13-1-15-31-22-8-6-20(7-9-22)24(28)19-4-5-19/h2-3,6-11,19,21,26H,1,4-5,12-15,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119720
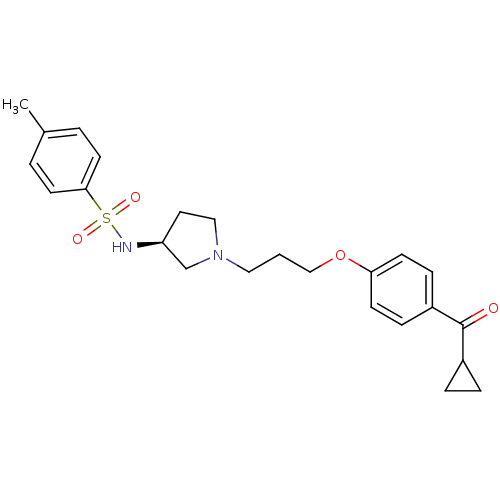
(CHEMBL107293 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-3-11-23(12-4-18)31(28,29)25-21-13-15-26(17-21)14-2-16-30-22-9-7-20(8-10-22)24(27)19-5-6-19/h3-4,7-12,19,21,25H,2,5-6,13-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119702

(CHEMBL102925 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES CCc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C25H32N2O4S/c1-2-19-4-12-24(13-5-19)32(29,30)26-22-14-16-27(18-22)15-3-17-31-23-10-8-21(9-11-23)25(28)20-6-7-20/h4-5,8-13,20,22,26H,2-3,6-7,14-18H2,1H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119736
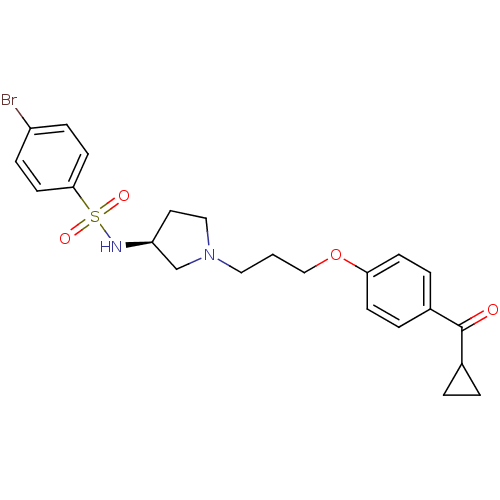
(4-Bromo-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES Brc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27BrN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119725

(4-tert-Butyl-N-{(S)-1-[3-(4-cyclopropanecarbonyl-p...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C27H36N2O4S/c1-27(2,3)22-9-13-25(14-10-22)34(31,32)28-23-15-17-29(19-23)16-4-18-33-24-11-7-21(8-12-24)26(30)20-5-6-20/h7-14,20,23,28H,4-6,15-19H2,1-3H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119733
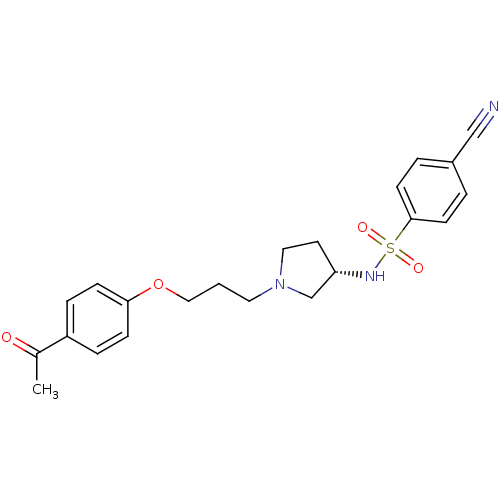
(CHEMBL102840 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C22H25N3O4S/c1-17(26)19-5-7-21(8-6-19)29-14-2-12-25-13-11-20(16-25)24-30(27,28)22-9-3-18(15-23)4-10-22/h3-10,20,24H,2,11-14,16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119698
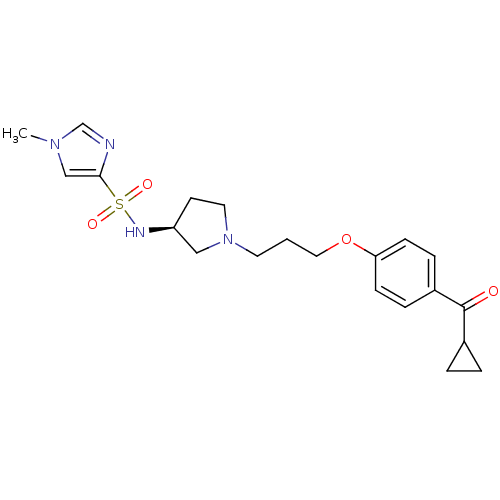
(1-Methyl-1H-imidazole-4-sulfonic acid {(S)-1-[3-(4...)Show SMILES Cn1cnc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C21H28N4O4S/c1-24-14-20(22-15-24)30(27,28)23-18-9-11-25(13-18)10-2-12-29-19-7-5-17(6-8-19)21(26)16-3-4-16/h5-8,14-16,18,23H,2-4,9-13H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119730
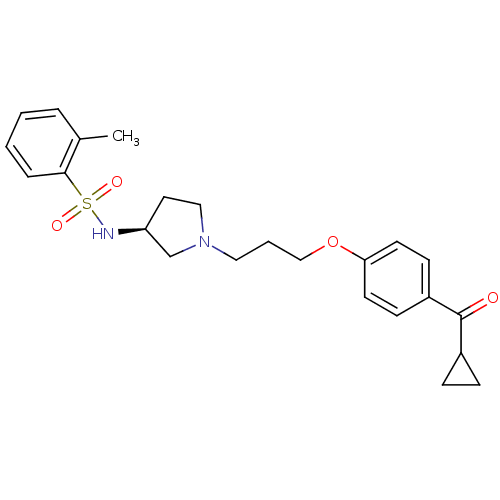
(CHEMBL102929 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-5-2-3-6-23(18)31(28,29)25-21-13-15-26(17-21)14-4-16-30-22-11-9-20(10-12-22)24(27)19-7-8-19/h2-3,5-6,9-12,19,21,25H,4,7-8,13-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119708
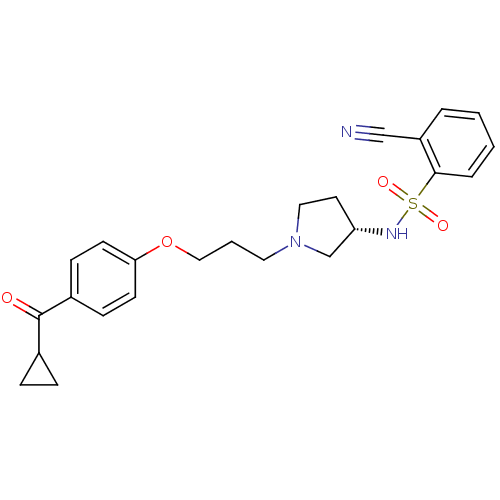
(2-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2C#N)cc1 Show InChI InChI=1S/C24H27N3O4S/c25-16-20-4-1-2-5-23(20)32(29,30)26-21-12-14-27(17-21)13-3-15-31-22-10-8-19(9-11-22)24(28)18-6-7-18/h1-2,4-5,8-11,18,21,26H,3,6-7,12-15,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50404002
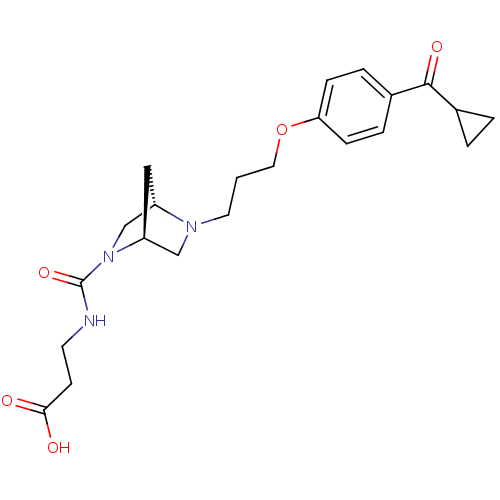
(CHEMBL2112452)Show SMILES OC(=O)CCNC(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1 Show InChI InChI=1S/C22H29N3O5/c26-20(27)8-9-23-22(29)25-14-17-12-18(25)13-24(17)10-1-11-30-19-6-4-16(5-7-19)21(28)15-2-3-15/h4-7,15,17-18H,1-3,8-14H2,(H,23,29)(H,26,27)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119739

(CHEMBL102794 | {4-[3-((1S,4S)-5-Benzenesulfonyl-2,...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2C[C@@H]3C[C@H]2CN3S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C24H28N2O4S/c27-24(18-7-8-18)19-9-11-22(12-10-19)30-14-4-13-25-16-21-15-20(25)17-26(21)31(28,29)23-5-2-1-3-6-23/h1-3,5-6,9-12,18,20-21H,4,7-8,13-17H2/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119703

(CHEMBL102331 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-16(25)17-5-7-20(8-6-17)27-13-3-11-24-12-9-19(15-24)23-21(26)18-4-2-10-22-14-18/h2,4-8,10,14,19H,3,9,11-13,15H2,1H3,(H,23,26)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119723
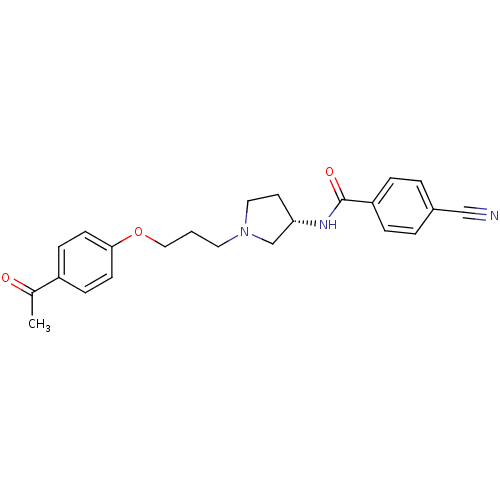
(CHEMBL430502 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C23H25N3O3/c1-17(27)19-7-9-22(10-8-19)29-14-2-12-26-13-11-21(16-26)25-23(28)20-5-3-18(15-24)4-6-20/h3-10,21H,2,11-14,16H2,1H3,(H,25,28)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119719
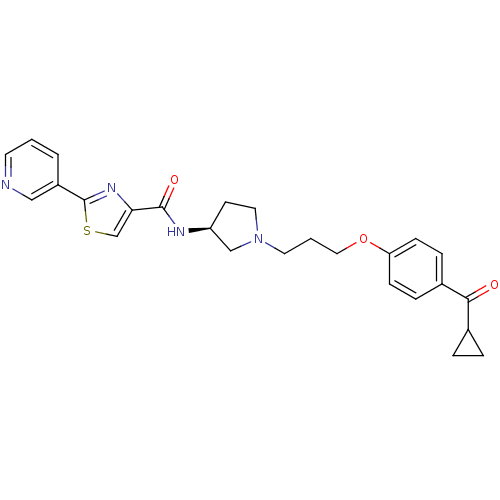
(2-Pyridin-3-yl-thiazole-4-carboxylic acid {(S)-1-[...)Show SMILES O=C(N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1)c1csc(n1)-c1cccnc1 Show InChI InChI=1S/C26H28N4O3S/c31-24(18-4-5-18)19-6-8-22(9-7-19)33-14-2-12-30-13-10-21(16-30)28-25(32)23-17-34-26(29-23)20-3-1-11-27-15-20/h1,3,6-9,11,15,17-18,21H,2,4-5,10,12-14,16H2,(H,28,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119706
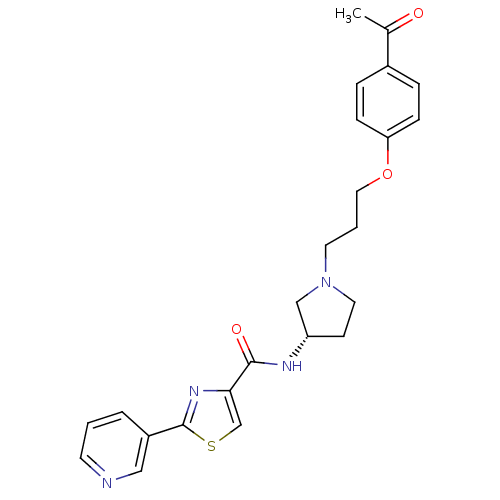
(2-Pyridin-3-yl-thiazole-4-carboxylic acid {(S)-1-[...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2csc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C24H26N4O3S/c1-17(29)18-5-7-21(8-6-18)31-13-3-11-28-12-9-20(15-28)26-23(30)22-16-32-24(27-22)19-4-2-10-25-14-19/h2,4-8,10,14,16,20H,3,9,11-13,15H2,1H3,(H,26,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119724

((3-{(1S,4S)-5-[3-(4-Cyclopropanecarbonyl-phenoxy)-...)Show SMILES CC(C)(C)OC(=O)NCCC(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1 Show InChI InChI=1S/C26H37N3O5/c1-26(2,3)34-25(32)27-12-11-23(30)29-17-20-15-21(29)16-28(20)13-4-14-33-22-9-7-19(8-10-22)24(31)18-5-6-18/h7-10,18,20-21H,4-6,11-17H2,1-3H3,(H,27,32)/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119734
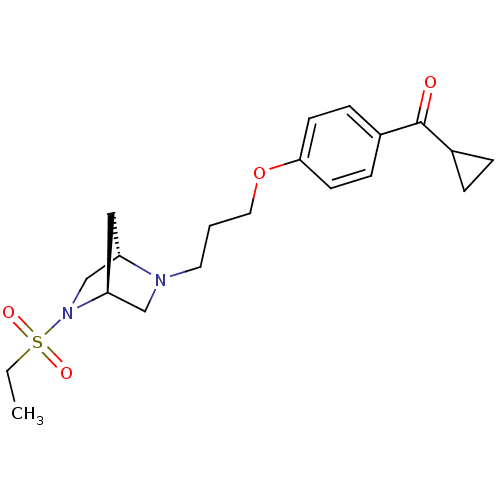
(CHEMBL102283 | Cyclopropyl-{4-[3-((1S,4S)-5-ethane...)Show SMILES CCS(=O)(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1 Show InChI InChI=1S/C20H28N2O4S/c1-2-27(24,25)22-14-17-12-18(22)13-21(17)10-3-11-26-19-8-6-16(7-9-19)20(23)15-4-5-15/h6-9,15,17-18H,2-5,10-14H2,1H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119740
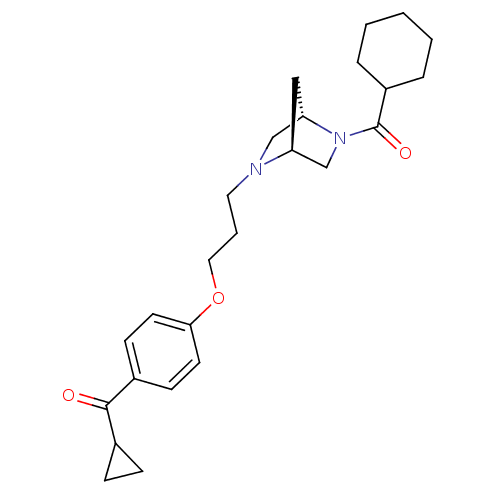
(CHEMBL419176 | {4-[3-((1S,4S)-5-Cyclohexanecarbony...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2C[C@@H]3C[C@H]2CN3C(=O)C2CCCCC2)cc1 Show InChI InChI=1S/C25H34N2O3/c28-24(18-7-8-18)19-9-11-23(12-10-19)30-14-4-13-26-16-22-15-21(26)17-27(22)25(29)20-5-2-1-3-6-20/h9-12,18,20-22H,1-8,13-17H2/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119713

((S)-N-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrroli...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@@H](N)CO)cc1 Show InChI InChI=1S/C18H27N3O4/c1-13(23)14-3-5-16(6-4-14)25-10-2-8-21-9-7-15(11-21)20-18(24)17(19)12-22/h3-6,15,17,22H,2,7-12,19H2,1H3,(H,20,24)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119697
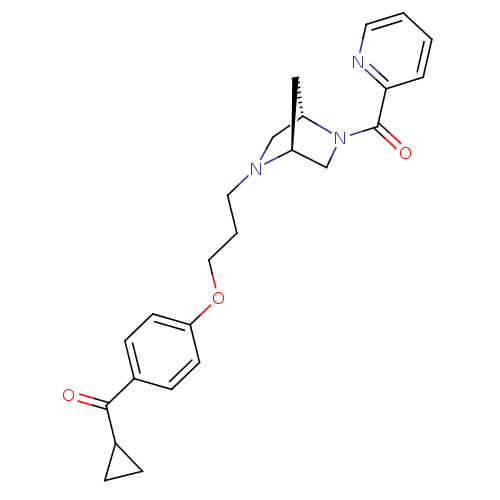
(CHEMBL105224 | Cyclopropyl-(4-{3-[(1S,4S)-5-(pyrid...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2C[C@@H]3C[C@H]2CN3C(=O)c2ccccn2)cc1 Show InChI InChI=1S/C24H27N3O3/c28-23(17-5-6-17)18-7-9-21(10-8-18)30-13-3-12-26-15-20-14-19(26)16-27(20)24(29)22-4-1-2-11-25-22/h1-2,4,7-11,17,19-20H,3,5-6,12-16H2/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119709

(CHEMBL322695 | Pyrazine-2-carboxylic acid {(S)-1-[...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2cnccn2)cc1 Show InChI InChI=1S/C20H24N4O3/c1-15(25)16-3-5-18(6-4-16)27-12-2-10-24-11-7-17(14-24)23-20(26)19-13-21-8-9-22-19/h3-6,8-9,13,17H,2,7,10-12,14H2,1H3,(H,23,26)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119729

(CHEMBL102853 | Cyclopropyl-(4-{3-[(1S,4S)-5-(furan...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2C[C@@H]3C[C@H]2CN3C(=O)c2ccco2)cc1 Show InChI InChI=1S/C23H26N2O4/c26-22(16-4-5-16)17-6-8-20(9-7-17)28-12-2-10-24-14-19-13-18(24)15-25(19)23(27)21-3-1-11-29-21/h1,3,6-9,11,16,18-19H,2,4-5,10,12-15H2/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119727

((S)-2-({(2S,7S)-5-[3-(4-Cyclopropanecarbonyl-pheno...)Show SMILES C[C@H](NC(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1)C(O)=O Show InChI InChI=1S/C22H29N3O5/c1-14(21(27)28)23-22(29)25-13-17-11-18(25)12-24(17)9-2-10-30-19-7-5-16(6-8-19)20(26)15-3-4-15/h5-8,14-15,17-18H,2-4,9-13H2,1H3,(H,23,29)(H,27,28)/t14-,17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119717
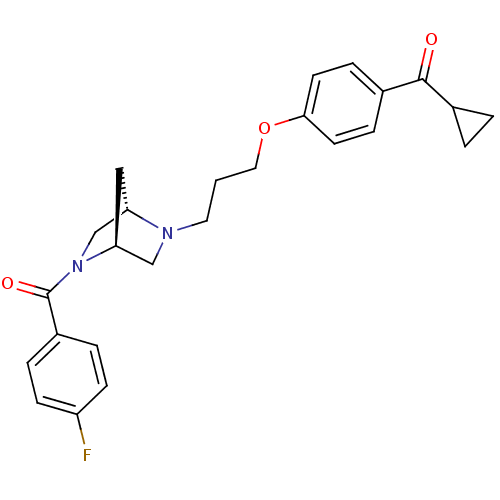
(CHEMBL320639 | Cyclopropyl-(4-{3-[(1S,4S)-5-(4-flu...)Show SMILES Fc1ccc(cc1)C(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1 Show InChI InChI=1S/C25H27FN2O3/c26-20-8-4-19(5-9-20)25(30)28-16-21-14-22(28)15-27(21)12-1-13-31-23-10-6-18(7-11-23)24(29)17-2-3-17/h4-11,17,21-22H,1-3,12-16H2/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119728

(4-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119736

(4-Bromo-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES Brc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27BrN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119726
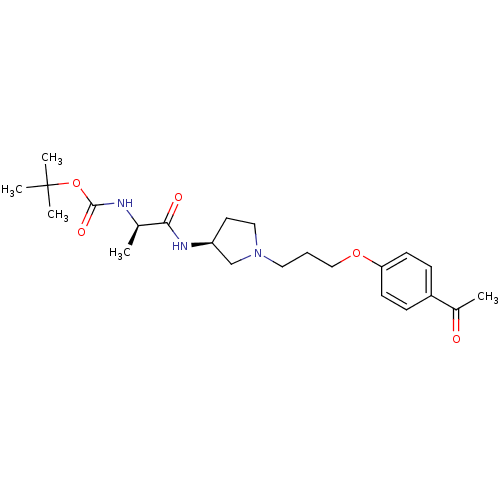
((1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrolidin...)Show SMILES C[C@@H](NC(=O)OC(C)(C)C)C(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(C)=O)C1 Show InChI InChI=1S/C23H35N3O5/c1-16(24-22(29)31-23(3,4)5)21(28)25-19-11-13-26(15-19)12-6-14-30-20-9-7-18(8-10-20)17(2)27/h7-10,16,19H,6,11-15H2,1-5H3,(H,24,29)(H,25,28)/t16-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 307 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119700
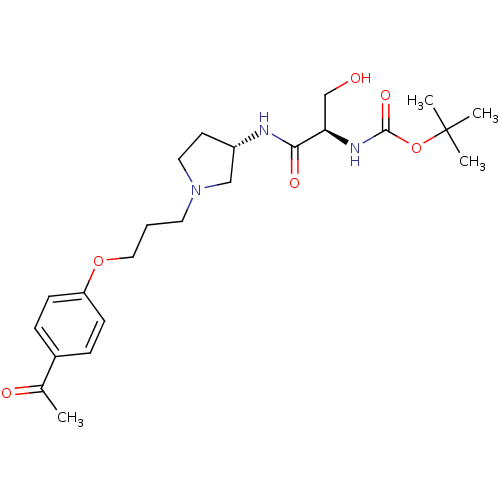
((1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrolidin...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@@H](CO)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C23H35N3O6/c1-16(28)17-6-8-19(9-7-17)31-13-5-11-26-12-10-18(14-26)24-21(29)20(15-27)25-22(30)32-23(2,3)4/h6-9,18,20,27H,5,10-15H2,1-4H3,(H,24,29)(H,25,30)/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119731

(2-(tert-Butoxycarbonyl-{(3S,6S)-5-[3-(4-cyclopropa...)Show SMILES C[C@@H](N(C(=O)OC(C)(C)C)C(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1)C(O)=O Show InChI InChI=1S/C27H37N3O7/c1-17(24(32)33)30(26(35)37-27(2,3)4)25(34)29-16-20-14-21(29)15-28(20)12-5-13-36-22-10-8-19(9-11-22)23(31)18-6-7-18/h8-11,17-18,20-21H,5-7,12-16H2,1-4H3,(H,32,33)/t17-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119702

(CHEMBL102925 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES CCc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C25H32N2O4S/c1-2-19-4-12-24(13-5-19)32(29,30)26-22-14-16-27(18-22)15-3-17-31-23-10-8-21(9-11-23)25(28)20-6-7-20/h4-5,8-13,20,22,26H,2-3,6-7,14-18H2,1H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119732

(CHEMBL318575 | {4-[3-((1S,4S)-5-Benzoyl-2,5-diaza-...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2C[C@@H]3C[C@H]2CN3C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H28N2O3/c28-24(18-7-8-18)19-9-11-23(12-10-19)30-14-4-13-26-16-22-15-21(26)17-27(22)25(29)20-5-2-1-3-6-20/h1-3,5-6,9-12,18,21-22H,4,7-8,13-17H2/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 344 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119720

(CHEMBL107293 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-3-11-23(12-4-18)31(28,29)25-21-13-15-26(17-21)14-2-16-30-22-9-7-20(8-10-22)24(27)19-5-6-19/h3-4,7-12,19,21,25H,2,5-6,13-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119714
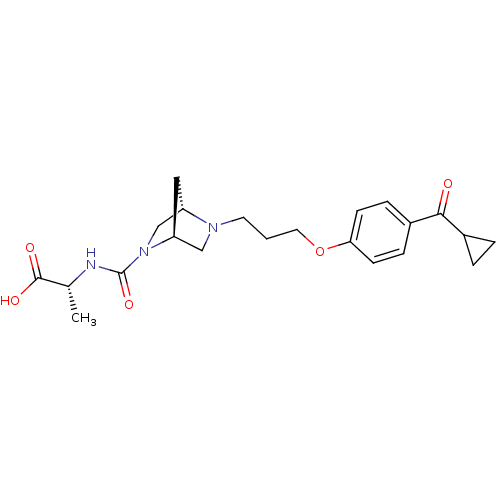
(2-({(2S,7S)-5-[3-(4-Cyclopropanecarbonyl-phenoxy)-...)Show SMILES C[C@@H](NC(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1)C(O)=O Show InChI InChI=1S/C22H29N3O5/c1-14(21(27)28)23-22(29)25-13-17-11-18(25)12-24(17)9-2-10-30-19-7-5-16(6-8-19)20(26)15-3-4-15/h5-8,14-15,17-18H,2-4,9-13H2,1H3,(H,23,29)(H,27,28)/t14-,17+,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 413 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119701

(CHEMBL104808 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119715
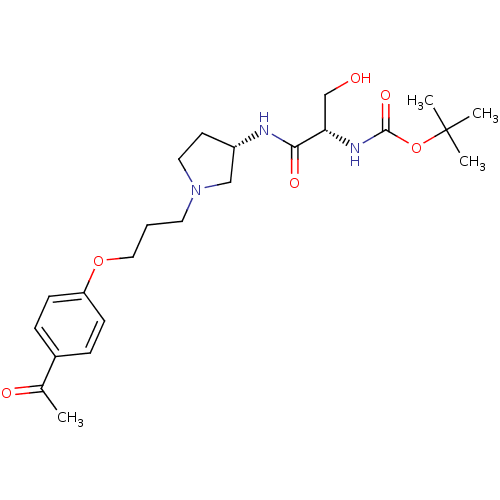
(((S)-1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrol...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@H](CO)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C23H35N3O6/c1-16(28)17-6-8-19(9-7-17)31-13-5-11-26-12-10-18(14-26)24-21(29)20(15-27)25-22(30)32-23(2,3)4/h6-9,18,20,27H,5,10-15H2,1-4H3,(H,24,29)(H,25,30)/t18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 587 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119696

(1-{4-[3-((S)-3-Amino-pyrrolidin-1-yl)-propoxy]-phe...)Show InChI InChI=1S/C15H22N2O2/c1-12(18)13-3-5-15(6-4-13)19-10-2-8-17-9-7-14(16)11-17/h3-6,14H,2,7-11,16H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards rat Histamine H3 receptor (For compound 11) |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119712

(3-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-19-3-1-4-22(15-19)31(28,29)25-20-11-13-26(16-20)12-2-14-30-21-9-7-18(8-10-21)23(27)17-5-6-17/h1,3-4,7-10,15,17,20,25H,2,5-6,11-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119708

(2-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2C#N)cc1 Show InChI InChI=1S/C24H27N3O4S/c25-16-20-4-1-2-5-23(20)32(29,30)26-21-12-14-27(17-21)13-3-15-31-22-10-8-19(9-11-22)24(28)18-6-7-18/h1-2,4-5,8-11,18,21,26H,3,6-7,12-15,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119725

(4-tert-Butyl-N-{(S)-1-[3-(4-cyclopropanecarbonyl-p...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C27H36N2O4S/c1-27(2,3)22-9-13-25(14-10-22)34(31,32)28-23-15-17-29(19-23)16-4-18-33-24-11-7-21(8-12-24)26(30)20-5-6-20/h7-14,20,23,28H,4-6,15-19H2,1-3H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119735

(3-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2cccc(c2)C#N)cc1 Show InChI InChI=1S/C24H27N3O4S/c25-16-18-3-1-4-23(15-18)32(29,30)26-21-11-13-27(17-21)12-2-14-31-22-9-7-20(8-10-22)24(28)19-5-6-19/h1,3-4,7-10,15,19,21,26H,2,5-6,11-14,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119718
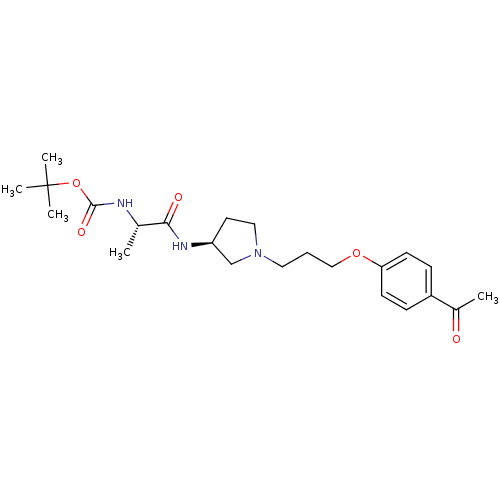
(((S)-1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrol...)Show SMILES C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(C)=O)C1 Show InChI InChI=1S/C23H35N3O5/c1-16(24-22(29)31-23(3,4)5)21(28)25-19-11-13-26(15-19)12-6-14-30-20-9-7-18(8-10-20)17(2)27/h7-10,16,19H,6,11-15H2,1-5H3,(H,24,29)(H,25,28)/t16-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 901 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119722

(CHEMBL107162 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O5S/c1-30-21-9-11-23(12-10-21)32(28,29)25-20-13-15-26(17-20)14-2-16-31-22-7-5-19(6-8-22)24(27)18-3-4-18/h5-12,18,20,25H,2-4,13-17H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119711

(2-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-21-4-1-2-5-22(21)31(28,29)25-19-12-14-26(16-19)13-3-15-30-20-10-8-18(9-11-20)23(27)17-6-7-17/h1-2,4-5,8-11,17,19,25H,3,6-7,12-16H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119705

(CHEMBL101691 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N2O4S/c26-23(18-7-8-18)19-9-11-21(12-10-19)29-16-4-14-25-15-13-20(17-25)24-30(27,28)22-5-2-1-3-6-22/h1-3,5-6,9-12,18,20,24H,4,7-8,13-17H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119736

(4-Bromo-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES Brc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27BrN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119710

(CHEMBL104618 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-21-4-1-2-5-22(21)31(28,29)25-19-12-14-26(16-19)13-3-15-30-20-10-8-18(9-11-20)23(27)17-6-7-17/h1-2,4-5,8-11,17,19,25H,3,6-7,12-16H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50404001

(CHEMBL2112451)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C24H27N3O4S/c25-16-18-2-10-23(11-3-18)32(29,30)26-21-12-14-27(17-21)13-1-15-31-22-8-6-20(7-9-22)24(28)19-4-5-19/h2-3,6-11,19,21,26H,1,4-5,12-15,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119704

(CHEMBL104809 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-19-3-1-4-22(15-19)31(28,29)25-20-11-13-26(16-20)12-2-14-30-21-9-7-18(8-10-21)23(27)17-5-6-17/h1,3-4,7-10,15,17,20,25H,2,5-6,11-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119696

(1-{4-[3-((S)-3-Amino-pyrrolidin-1-yl)-propoxy]-phe...)Show InChI InChI=1S/C15H22N2O2/c1-12(18)13-3-5-15(6-4-13)19-10-2-8-17-9-7-14(16)11-17/h3-6,14H,2,7-11,16H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119721

(CHEMBL103027 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-4-2-5-23(16-18)31(28,29)25-21-12-14-26(17-21)13-3-15-30-22-10-8-20(9-11-22)24(27)19-6-7-19/h2,4-5,8-11,16,19,21,25H,3,6-7,12-15,17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119737
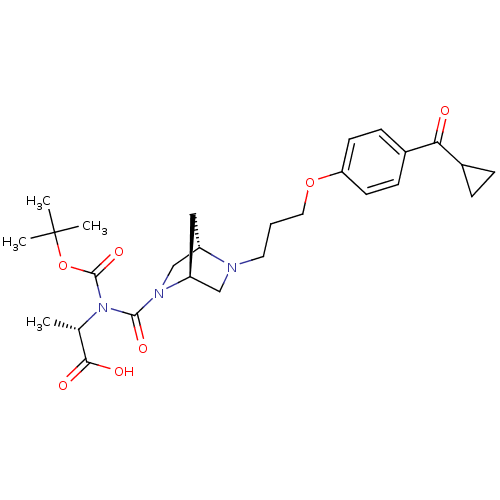
((S)-2-(tert-Butoxycarbonyl-{(3S,6S)-5-[3-(4-cyclop...)Show SMILES C[C@H](N(C(=O)OC(C)(C)C)C(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1)C(O)=O Show InChI InChI=1S/C27H37N3O7/c1-17(24(32)33)30(26(35)37-27(2,3)4)25(34)29-16-20-14-21(29)15-28(20)12-5-13-36-22-10-8-19(9-11-22)23(31)18-6-7-18/h8-11,17-18,20-21H,5-7,12-16H2,1-4H3,(H,32,33)/t17-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119730

(CHEMBL102929 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-5-2-3-6-23(18)31(28,29)25-21-13-15-26(17-21)14-4-16-30-22-11-9-20(10-12-22)24(27)19-7-8-19/h2-3,5-6,9-12,19,21,25H,4,7-8,13-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119725

(4-tert-Butyl-N-{(S)-1-[3-(4-cyclopropanecarbonyl-p...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C27H36N2O4S/c1-27(2,3)22-9-13-25(14-10-22)34(31,32)28-23-15-17-29(19-23)16-4-18-33-24-11-7-21(8-12-24)26(30)20-5-6-20/h7-14,20,23,28H,4-6,15-19H2,1-3H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119733

(CHEMBL102840 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C22H25N3O4S/c1-17(26)19-5-7-21(8-6-19)29-14-2-12-25-13-11-20(16-25)24-30(27,28)22-9-3-18(15-23)4-10-22/h3-10,20,24H,2,11-14,16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119702

(CHEMBL102925 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES CCc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C25H32N2O4S/c1-2-19-4-12-24(13-5-19)32(29,30)26-22-14-16-27(18-22)15-3-17-31-23-10-8-21(9-11-23)25(28)20-6-7-20/h4-5,8-13,20,22,26H,2-3,6-7,14-18H2,1H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119728

(4-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119703

(CHEMBL102331 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-16(25)17-5-7-20(8-6-17)27-13-3-11-24-12-9-19(15-24)23-21(26)18-4-2-10-22-14-18/h2,4-8,10,14,19H,3,9,11-13,15H2,1H3,(H,23,26)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119712

(3-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-19-3-1-4-22(15-19)31(28,29)25-20-11-13-26(16-20)12-2-14-30-21-9-7-18(8-10-21)23(27)17-5-6-17/h1,3-4,7-10,15,17,20,25H,2,5-6,11-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119719

(2-Pyridin-3-yl-thiazole-4-carboxylic acid {(S)-1-[...)Show SMILES O=C(N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1)c1csc(n1)-c1cccnc1 Show InChI InChI=1S/C26H28N4O3S/c31-24(18-4-5-18)19-6-8-22(9-7-19)33-14-2-12-30-13-10-21(16-30)28-25(32)23-17-34-26(29-23)20-3-1-11-27-15-20/h1,3,6-9,11,15,17-18,21H,2,4-5,10,12-14,16H2,(H,28,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119722

(CHEMBL107162 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O5S/c1-30-21-9-11-23(12-10-21)32(28,29)25-20-13-15-26(17-20)14-2-16-31-22-7-5-19(6-8-22)24(27)18-3-4-18/h5-12,18,20,25H,2-4,13-17H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119711

(2-Chloro-N-{(S)-1-[3-(4-cyclopropanecarbonyl-pheno...)Show SMILES Clc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27ClN2O4S/c24-21-4-1-2-5-22(21)31(28,29)25-19-12-14-26(16-19)13-3-15-30-20-10-8-18(9-11-20)23(27)17-6-7-17/h1-2,4-5,8-11,17,19,25H,3,6-7,12-16H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119709

(CHEMBL322695 | Pyrazine-2-carboxylic acid {(S)-1-[...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2cnccn2)cc1 Show InChI InChI=1S/C20H24N4O3/c1-15(25)16-3-5-18(6-4-16)27-12-2-10-24-11-7-17(14-24)23-20(26)19-13-21-8-9-22-19/h3-6,8-9,13,17H,2,7,10-12,14H2,1H3,(H,23,26)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119720

(CHEMBL107293 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-3-11-23(12-4-18)31(28,29)25-21-13-15-26(17-21)14-2-16-30-22-9-7-20(8-10-22)24(27)19-5-6-19/h3-4,7-12,19,21,25H,2,5-6,13-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119704

(CHEMBL104809 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-19-3-1-4-22(15-19)31(28,29)25-20-11-13-26(16-20)12-2-14-30-21-9-7-18(8-10-21)23(27)17-5-6-17/h1,3-4,7-10,15,17,20,25H,2,5-6,11-14,16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119723

(CHEMBL430502 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C23H25N3O3/c1-17(27)19-7-9-22(10-8-19)29-14-2-12-26-13-11-21(16-26)25-23(28)20-5-3-18(15-24)4-6-20/h3-10,21H,2,11-14,16H2,1H3,(H,25,28)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119706

(2-Pyridin-3-yl-thiazole-4-carboxylic acid {(S)-1-[...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2csc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C24H26N4O3S/c1-17(29)18-5-7-21(8-6-18)31-13-3-11-28-12-9-20(15-28)26-23(30)22-16-32-24(27-22)19-4-2-10-25-14-19/h2,4-8,10,14,16,20H,3,9,11-13,15H2,1H3,(H,26,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119708

(2-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2C#N)cc1 Show InChI InChI=1S/C24H27N3O4S/c25-16-20-4-1-2-5-23(20)32(29,30)26-21-12-14-27(17-21)13-3-15-31-22-10-8-19(9-11-22)24(28)18-6-7-18/h1-2,4-5,8-11,18,21,26H,3,6-7,12-15,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119721

(CHEMBL103027 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1cccc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-4-2-5-23(16-18)31(28,29)25-21-12-14-26(17-21)13-3-15-30-22-10-8-20(9-11-22)24(27)19-6-7-19/h2,4-5,8-11,16,19,21,25H,3,6-7,12-15,17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119698

(1-Methyl-1H-imidazole-4-sulfonic acid {(S)-1-[3-(4...)Show SMILES Cn1cnc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C21H28N4O4S/c1-24-14-20(22-15-24)30(27,28)23-18-9-11-25(13-18)10-2-12-29-19-7-5-17(6-8-19)21(26)16-3-4-16/h5-8,14-16,18,23H,2-4,9-13H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119703

(CHEMBL102331 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2cccnc2)cc1 Show InChI InChI=1S/C21H25N3O3/c1-16(25)17-5-7-20(8-6-17)27-13-3-11-24-12-9-19(15-24)23-21(26)18-4-2-10-22-14-18/h2,4-8,10,14,19H,3,9,11-13,15H2,1H3,(H,23,26)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119735

(3-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenox...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2cccc(c2)C#N)cc1 Show InChI InChI=1S/C24H27N3O4S/c25-16-18-3-1-4-23(15-18)32(29,30)26-21-11-13-27(17-21)12-2-14-31-22-9-7-20(8-10-22)24(28)19-5-6-19/h1,3-4,7-10,15,19,21,26H,2,5-6,11-14,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119705

(CHEMBL101691 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C23H28N2O4S/c26-23(18-7-8-18)19-9-11-21(12-10-19)29-16-4-14-25-15-13-20(17-25)24-30(27,28)22-5-2-1-3-6-22/h1-3,5-6,9-12,18,20,24H,4,7-8,13-17H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119701

(CHEMBL104808 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1ccc(cc1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-19-6-10-22(11-7-19)31(28,29)25-20-12-14-26(16-20)13-1-15-30-21-8-4-18(5-9-21)23(27)17-2-3-17/h4-11,17,20,25H,1-3,12-16H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119710

(CHEMBL104618 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Fc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C23H27FN2O4S/c24-21-4-1-2-5-22(21)31(28,29)25-19-12-14-26(16-19)13-3-15-30-20-10-8-18(9-11-20)23(27)17-6-7-17/h1-2,4-5,8-11,17,19,25H,3,6-7,12-16H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119709

(CHEMBL322695 | Pyrazine-2-carboxylic acid {(S)-1-[...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2cnccn2)cc1 Show InChI InChI=1S/C20H24N4O3/c1-15(25)16-3-5-18(6-4-16)27-12-2-10-24-11-7-17(14-24)23-20(26)19-13-21-8-9-22-19/h3-6,8-9,13,17H,2,7,10-12,14H2,1H3,(H,23,26)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50119738

(1-{(1S,4S)-5-[3-(4-Cyclopropanecarbonyl-phenoxy)-p...)Show SMILES CC(C)(C)CC(=O)N1C[C@@H]2C[C@H]1CN2CCCOc1ccc(cc1)C(=O)C1CC1 Show InChI InChI=1S/C24H34N2O3/c1-24(2,3)14-22(27)26-16-19-13-20(26)15-25(19)11-4-12-29-21-9-7-18(8-10-21)23(28)17-5-6-17/h7-10,17,19-20H,4-6,11-16H2,1-3H3/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against rat histamine H3 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119706

(2-Pyridin-3-yl-thiazole-4-carboxylic acid {(S)-1-[...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2csc(n2)-c2cccnc2)cc1 Show InChI InChI=1S/C24H26N4O3S/c1-17(29)18-5-7-21(8-6-18)31-13-3-11-28-12-9-20(15-28)26-23(30)22-16-32-24(27-22)19-4-2-10-25-14-19/h2,4-8,10,14,16,20H,3,9,11-13,15H2,1H3,(H,26,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119723

(CHEMBL430502 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C23H25N3O3/c1-17(27)19-7-9-22(10-8-19)29-14-2-12-26-13-11-21(16-26)25-23(28)20-5-3-18(15-24)4-6-20/h3-10,21H,2,11-14,16H2,1H3,(H,25,28)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119733

(CHEMBL102840 | N-{(S)-1-[3-(4-Acetyl-phenoxy)-prop...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccc(cc2)C#N)cc1 Show InChI InChI=1S/C22H25N3O4S/c1-17(26)19-5-7-21(8-6-19)29-14-2-12-25-13-11-20(16-25)24-30(27,28)22-9-3-18(15-23)4-10-22/h3-10,20,24H,2,11-14,16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119730

(CHEMBL102929 | N-{(S)-1-[3-(4-Cyclopropanecarbonyl...)Show SMILES Cc1ccccc1S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C24H30N2O4S/c1-18-5-2-3-6-23(18)31(28,29)25-21-13-15-26(17-21)14-4-16-30-22-11-9-20(10-12-22)24(27)19-7-8-19/h2-3,5-6,9-12,19,21,25H,4,7-8,13-17H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50404001

(CHEMBL2112451)Show SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C24H27N3O4S/c25-16-18-2-10-23(11-3-18)32(29,30)26-21-12-14-27(17-21)13-1-15-31-22-8-6-20(7-9-22)24(28)19-4-5-19/h2-3,6-11,19,21,26H,1,4-5,12-15,17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119713

((S)-N-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrroli...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@@H](N)CO)cc1 Show InChI InChI=1S/C18H27N3O4/c1-13(23)14-3-5-16(6-4-14)25-10-2-8-21-9-7-15(11-21)20-18(24)17(19)12-22/h3-6,15,17,22H,2,7-12,19H2,1H3,(H,20,24)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119718

(((S)-1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrol...)Show SMILES C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(C)=O)C1 Show InChI InChI=1S/C23H35N3O5/c1-16(24-22(29)31-23(3,4)5)21(28)25-19-11-13-26(15-19)12-6-14-30-20-9-7-18(8-10-20)17(2)27/h7-10,16,19H,6,11-15H2,1-5H3,(H,24,29)(H,25,28)/t16-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119696

(1-{4-[3-((S)-3-Amino-pyrrolidin-1-yl)-propoxy]-phe...)Show InChI InChI=1S/C15H22N2O2/c1-12(18)13-3-5-15(6-4-13)19-10-2-8-17-9-7-14(16)11-17/h3-6,14H,2,7-11,16H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Histamine H2 receptor (For compound 11) |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119719

(2-Pyridin-3-yl-thiazole-4-carboxylic acid {(S)-1-[...)Show SMILES O=C(N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1)c1csc(n1)-c1cccnc1 Show InChI InChI=1S/C26H28N4O3S/c31-24(18-4-5-18)19-6-8-22(9-7-19)33-14-2-12-30-13-10-21(16-30)28-25(32)23-17-34-26(29-23)20-3-1-11-27-15-20/h1,3,6-9,11,15,17-18,21H,2,4-5,10,12-14,16H2,(H,28,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119726

((1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrolidin...)Show SMILES C[C@@H](NC(=O)OC(C)(C)C)C(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(C)=O)C1 Show InChI InChI=1S/C23H35N3O5/c1-16(24-22(29)31-23(3,4)5)21(28)25-19-11-13-26(15-19)12-6-14-30-20-9-7-18(8-10-20)17(2)27/h7-10,16,19H,6,11-15H2,1-5H3,(H,24,29)(H,25,28)/t16-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119713

((S)-N-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrroli...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@@H](N)CO)cc1 Show InChI InChI=1S/C18H27N3O4/c1-13(23)14-3-5-16(6-4-14)25-10-2-8-21-9-7-15(11-21)20-18(24)17(19)12-22/h3-6,15,17,22H,2,7-12,19H2,1H3,(H,20,24)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119715

(((S)-1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrol...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@H](CO)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C23H35N3O6/c1-16(28)17-6-8-19(9-7-17)31-13-5-11-26-12-10-18(14-26)24-21(29)20(15-27)25-22(30)32-23(2,3)4/h6-9,18,20,27H,5,10-15H2,1-4H3,(H,24,29)(H,25,30)/t18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119696

(1-{4-[3-((S)-3-Amino-pyrrolidin-1-yl)-propoxy]-phe...)Show InChI InChI=1S/C15H22N2O2/c1-12(18)13-3-5-15(6-4-13)19-10-2-8-17-9-7-14(16)11-17/h3-6,14H,2,7-11,16H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119726

((1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrolidin...)Show SMILES C[C@@H](NC(=O)OC(C)(C)C)C(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(C)=O)C1 Show InChI InChI=1S/C23H35N3O5/c1-16(24-22(29)31-23(3,4)5)21(28)25-19-11-13-26(15-19)12-6-14-30-20-9-7-18(8-10-20)17(2)27/h7-10,16,19H,6,11-15H2,1-5H3,(H,24,29)(H,25,28)/t16-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119718

(((S)-1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrol...)Show SMILES C[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(C)=O)C1 Show InChI InChI=1S/C23H35N3O5/c1-16(24-22(29)31-23(3,4)5)21(28)25-19-11-13-26(15-19)12-6-14-30-20-9-7-18(8-10-20)17(2)27/h7-10,16,19H,6,11-15H2,1-5H3,(H,24,29)(H,25,28)/t16-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119715

(((S)-1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrol...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@H](CO)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C23H35N3O6/c1-16(28)17-6-8-19(9-7-17)31-13-5-11-26-12-10-18(14-26)24-21(29)20(15-27)25-22(30)32-23(2,3)4/h6-9,18,20,27H,5,10-15H2,1-4H3,(H,24,29)(H,25,30)/t18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119700

((1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrolidin...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@@H](CO)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C23H35N3O6/c1-16(28)17-6-8-19(9-7-17)31-13-5-11-26-12-10-18(14-26)24-21(29)20(15-27)25-22(30)32-23(2,3)4/h6-9,18,20,27H,5,10-15H2,1-4H3,(H,24,29)(H,25,30)/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119696

(1-{4-[3-((S)-3-Amino-pyrrolidin-1-yl)-propoxy]-phe...)Show InChI InChI=1S/C15H22N2O2/c1-12(18)13-3-5-15(6-4-13)19-10-2-8-17-9-7-14(16)11-17/h3-6,14H,2,7-11,16H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119698

(1-Methyl-1H-imidazole-4-sulfonic acid {(S)-1-[3-(4...)Show SMILES Cn1cnc(c1)S(=O)(=O)N[C@H]1CCN(CCCOc2ccc(cc2)C(=O)C2CC2)C1 Show InChI InChI=1S/C21H28N4O4S/c1-24-14-20(22-15-24)30(27,28)23-18-9-11-25(13-18)10-2-12-29-19-7-5-17(6-8-19)21(26)16-3-4-16/h5-8,14-16,18,23H,2-4,9-13H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H1 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50119700

((1-{(S)-1-[3-(4-Acetyl-phenoxy)-propyl]-pyrrolidin...)Show SMILES CC(=O)c1ccc(OCCCN2CC[C@@H](C2)NC(=O)[C@@H](CO)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C23H35N3O6/c1-16(28)17-6-8-19(9-7-17)31-13-5-11-26-12-10-18(14-26)24-21(29)20(15-27)25-22(30)32-23(2,3)4/h6-9,18,20,27H,5,10-15H2,1-4H3,(H,24,29)(H,25,30)/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to the human Histamine H2 receptor |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50119696

(1-{4-[3-((S)-3-Amino-pyrrolidin-1-yl)-propoxy]-phe...)Show InChI InChI=1S/C15H22N2O2/c1-12(18)13-3-5-15(6-4-13)19-10-2-8-17-9-7-14(16)11-17/h3-6,14H,2,7-11,16H2,1H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human Histamine H1 receptor (For compound 11) |
Bioorg Med Chem Lett 12: 3055-8 (2002)
BindingDB Entry DOI: 10.7270/Q2B56J3P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data