 Found 141 hits of Enzyme Inhibition Constant Data
Found 141 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153127
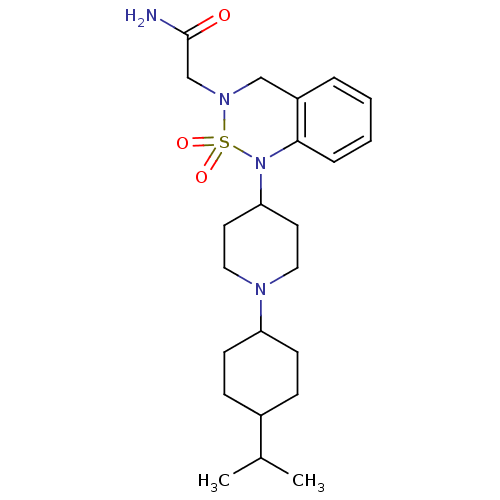
(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153124
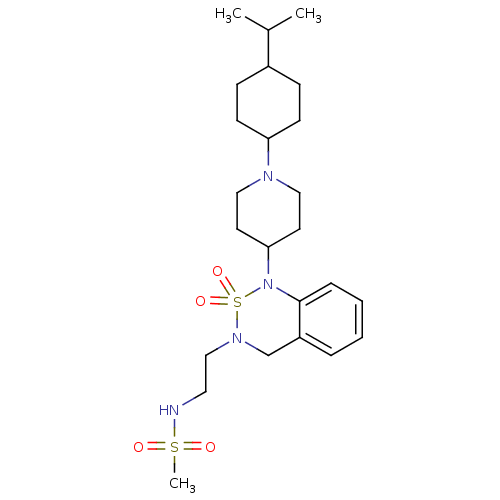
(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153121
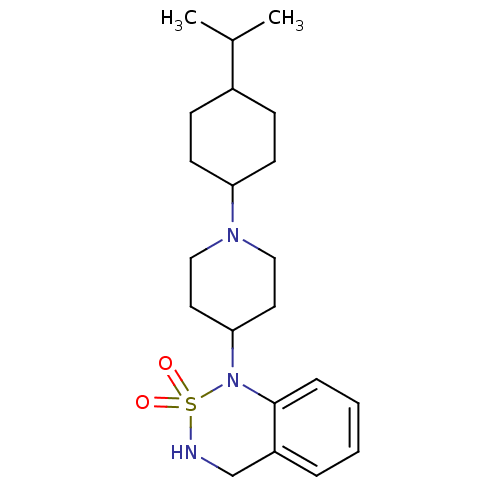
(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153132
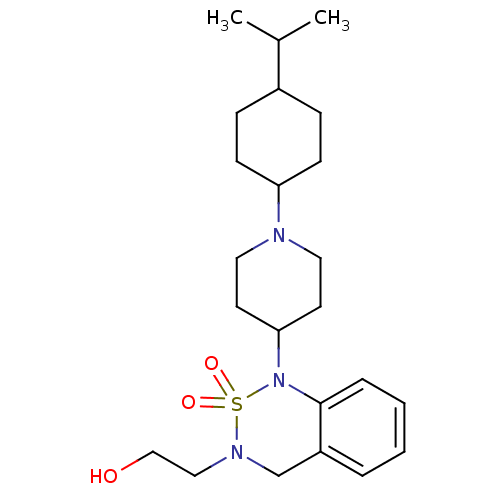
(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153135
(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153131
(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153131

(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153123
(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153125
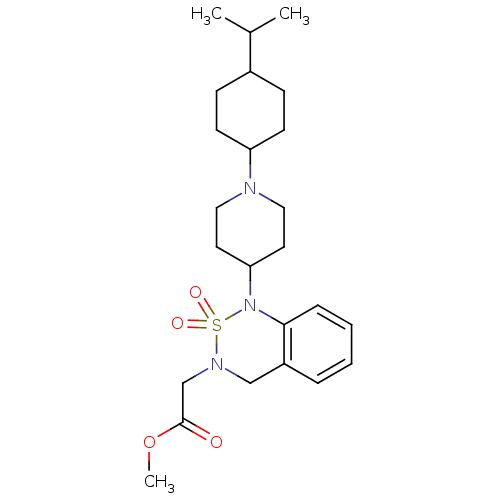
(CHEMBL364844 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES COC(=O)CN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.13,4.69,;4.78,5.41,;3.47,4.62,;3.51,3.08,;2.11,5.35,;.8,4.55,;-.55,5.3,;-1.84,4.48,;-3.19,5.23,;-4.5,4.43,;-4.46,2.89,;-3.12,2.14,;-1.82,2.94,;-.46,2.24,;-.44,.69,;-1.74,-.1,;-1.72,-1.67,;-.37,-2.38,;.94,-1.6,;.92,-.05,;-.37,-3.93,;-1.7,-4.71,;-1.7,-6.25,;-.37,-7.02,;.97,-6.25,;.97,-4.71,;-.34,-8.56,;-1.67,-9.35,;1.01,-9.33,;.85,3.03,;2.32,3.43,;1.25,1.53,)| Show InChI InChI=1S/C24H37N3O4S/c1-18(2)19-8-10-21(11-9-19)25-14-12-22(13-15-25)27-23-7-5-4-6-20(23)16-26(32(27,29)30)17-24(28)31-3/h4-7,18-19,21-22H,8-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153141
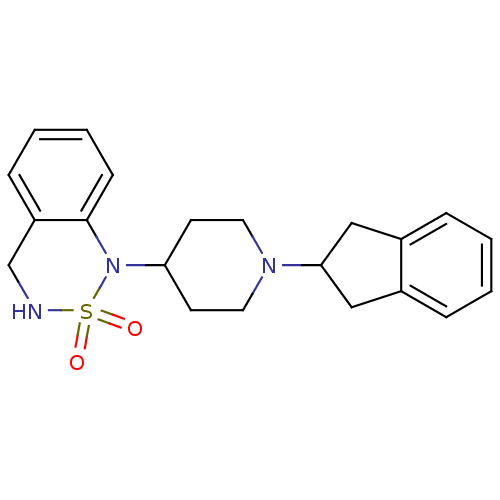
(1-(1-Indan-2-yl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1Cc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-15-18-7-3-4-8-21(18)24(27)19-9-11-23(12-10-19)20-13-16-5-1-2-6-17(16)14-20/h1-8,19-20,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153122
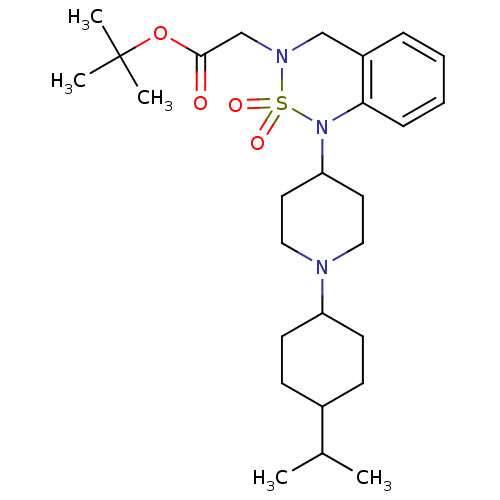
(CHEMBL181545 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(=O)OC(C)(C)C)S1(=O)=O |(-2.24,-9.73,;-.9,-8.94,;.44,-9.71,;-.93,-7.4,;-2.26,-6.62,;-2.26,-5.08,;-.93,-4.31,;.41,-5.08,;.41,-6.62,;-.94,-2.77,;-2.28,-2.04,;-2.31,-.48,;-1,.32,;.36,-.43,;.38,-1.97,;-1.02,1.86,;-2.38,2.56,;-3.69,1.77,;-5.02,2.52,;-5.07,4.06,;-3.76,4.86,;-2.4,4.11,;-1.11,4.9,;.24,4.18,;1.55,4.98,;2.91,4.25,;2.95,2.7,;4.22,5.04,;5.57,4.32,;4.85,2.96,;6.91,3.55,;6.34,5.65,;.29,2.66,;1.76,3.06,;.68,1.16,)| Show InChI InChI=1S/C27H43N3O4S/c1-20(2)21-10-12-23(13-11-21)28-16-14-24(15-17-28)30-25-9-7-6-8-22(25)18-29(35(30,32)33)19-26(31)34-27(3,4)5/h6-9,20-21,23-24H,10-19H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153123

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153135

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153132

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153127

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153124

(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153125

(CHEMBL364844 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES COC(=O)CN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.13,4.69,;4.78,5.41,;3.47,4.62,;3.51,3.08,;2.11,5.35,;.8,4.55,;-.55,5.3,;-1.84,4.48,;-3.19,5.23,;-4.5,4.43,;-4.46,2.89,;-3.12,2.14,;-1.82,2.94,;-.46,2.24,;-.44,.69,;-1.74,-.1,;-1.72,-1.67,;-.37,-2.38,;.94,-1.6,;.92,-.05,;-.37,-3.93,;-1.7,-4.71,;-1.7,-6.25,;-.37,-7.02,;.97,-6.25,;.97,-4.71,;-.34,-8.56,;-1.67,-9.35,;1.01,-9.33,;.85,3.03,;2.32,3.43,;1.25,1.53,)| Show InChI InChI=1S/C24H37N3O4S/c1-18(2)19-8-10-21(11-9-19)25-14-12-22(13-15-25)27-23-7-5-4-6-20(23)16-26(32(27,29)30)17-24(28)31-3/h4-7,18-19,21-22H,8-17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153128
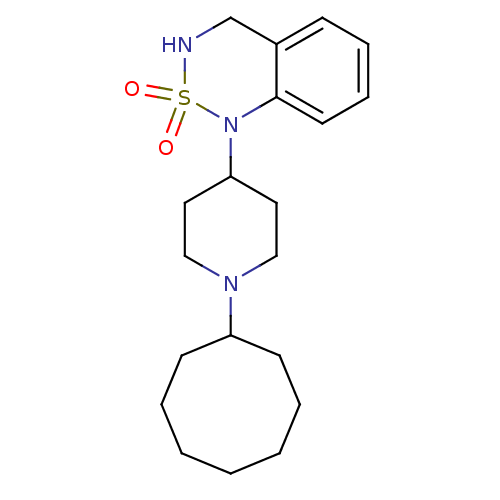
(1-(1-Cyclooctyl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show InChI InChI=1S/C20H31N3O2S/c24-26(25)21-16-17-8-6-7-11-20(17)23(26)19-12-14-22(15-13-19)18-9-4-2-1-3-5-10-18/h6-8,11,18-19,21H,1-5,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153134
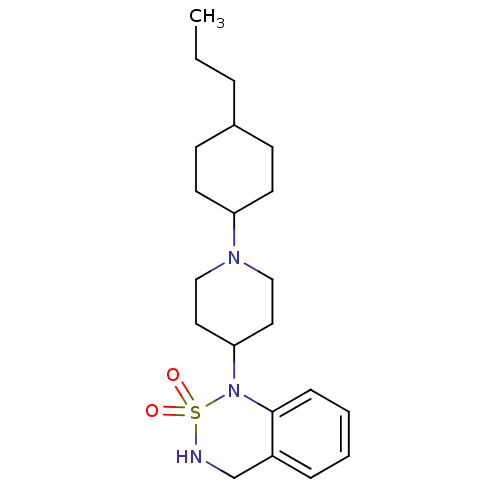
(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-3,4-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(9.9,-1.55,;8.44,-1.05,;7.29,-2.09,;5.82,-1.6,;4.68,-2.62,;3.22,-2.13,;2.92,-.62,;4.05,.4,;5.52,-.09,;1.46,-.14,;.31,-1.18,;-1.14,-.68,;-1.45,.82,;-.32,1.84,;1.15,1.35,;-2.91,1.28,;-3.89,.09,;-3.36,-1.34,;-4.31,-2.53,;-5.86,-2.27,;-6.39,-.85,;-5.41,.35,;-5.97,1.8,;-4.99,2.99,;-3.45,2.73,;-1.93,2.87,;-3.59,4.27,)| Show InChI InChI=1S/C21H33N3O2S/c1-2-5-17-8-10-19(11-9-17)23-14-12-20(13-15-23)24-21-7-4-3-6-18(21)16-22-27(24,25)26/h3-4,6-7,17,19-20,22H,2,5,8-16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 207 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153126
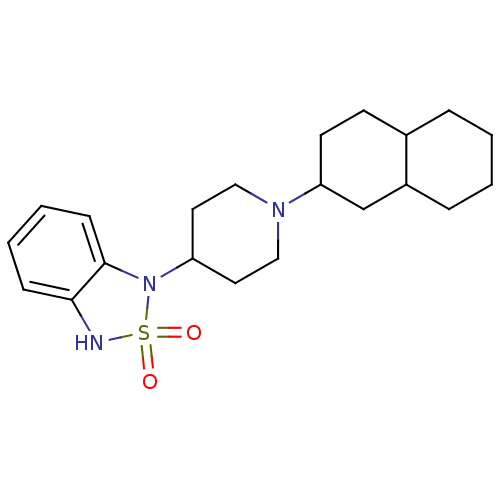
(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-1...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C21H31N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h3-4,7-8,16-19,22H,1-2,5-6,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153134

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-3,4-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(9.9,-1.55,;8.44,-1.05,;7.29,-2.09,;5.82,-1.6,;4.68,-2.62,;3.22,-2.13,;2.92,-.62,;4.05,.4,;5.52,-.09,;1.46,-.14,;.31,-1.18,;-1.14,-.68,;-1.45,.82,;-.32,1.84,;1.15,1.35,;-2.91,1.28,;-3.89,.09,;-3.36,-1.34,;-4.31,-2.53,;-5.86,-2.27,;-6.39,-.85,;-5.41,.35,;-5.97,1.8,;-4.99,2.99,;-3.45,2.73,;-1.93,2.87,;-3.59,4.27,)| Show InChI InChI=1S/C21H33N3O2S/c1-2-5-17-8-10-19(11-9-17)23-14-12-20(13-15-23)24-21-7-4-3-6-18(21)16-22-27(24,25)26/h3-4,6-7,17,19-20,22H,2,5,8-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153122

(CHEMBL181545 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(=O)OC(C)(C)C)S1(=O)=O |(-2.24,-9.73,;-.9,-8.94,;.44,-9.71,;-.93,-7.4,;-2.26,-6.62,;-2.26,-5.08,;-.93,-4.31,;.41,-5.08,;.41,-6.62,;-.94,-2.77,;-2.28,-2.04,;-2.31,-.48,;-1,.32,;.36,-.43,;.38,-1.97,;-1.02,1.86,;-2.38,2.56,;-3.69,1.77,;-5.02,2.52,;-5.07,4.06,;-3.76,4.86,;-2.4,4.11,;-1.11,4.9,;.24,4.18,;1.55,4.98,;2.91,4.25,;2.95,2.7,;4.22,5.04,;5.57,4.32,;4.85,2.96,;6.91,3.55,;6.34,5.65,;.29,2.66,;1.76,3.06,;.68,1.16,)| Show InChI InChI=1S/C27H43N3O4S/c1-20(2)21-10-12-23(13-11-21)28-16-14-24(15-17-28)30-25-9-7-6-8-22(25)18-29(35(30,32)33)19-26(31)34-27(3,4)5/h6-9,20-21,23-24H,10-19H2,1-5H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153148
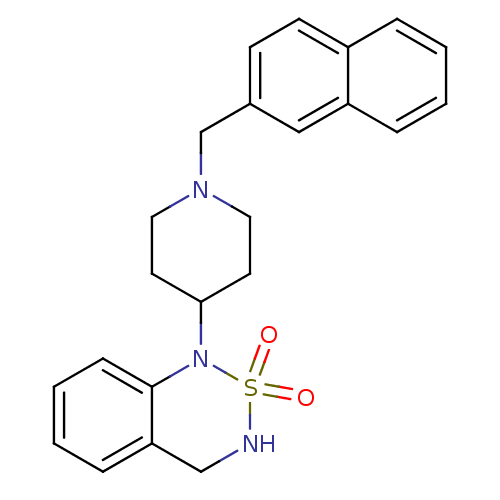
(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-29(28)24-16-21-7-3-4-8-23(21)26(29)22-11-13-25(14-12-22)17-18-9-10-19-5-1-2-6-20(19)15-18/h1-10,15,22,24H,11-14,16-17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153130
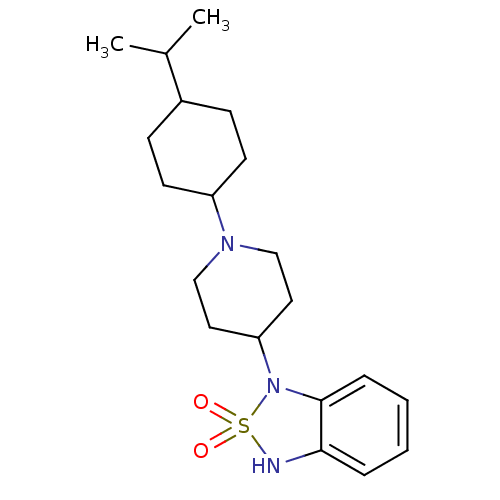
(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(7.16,-4.52,;7.03,-3.01,;8.3,-2.12,;5.65,-2.33,;4.36,-3.23,;2.95,-2.57,;2.84,-1.01,;4.1,-.12,;5.52,-.8,;1.42,-.33,;.15,-1.22,;-1.23,-.54,;-1.36,.99,;-.09,1.86,;1.3,1.2,;-2.74,1.64,;-4.02,.83,;-4.3,-.68,;-5.73,-1.19,;-6.92,-.19,;-6.65,1.3,;-5.21,1.83,;-4.62,3.25,;-3.09,3.13,;-1.6,3.41,;-3.39,4.62,)| Show InChI InChI=1S/C20H31N3O2S/c1-15(2)16-7-9-17(10-8-16)22-13-11-18(12-14-22)23-20-6-4-3-5-19(20)21-26(23,24)25/h3-6,15-18,21H,7-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153148

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-29(28)24-16-21-7-3-4-8-23(21)26(29)22-11-13-25(14-12-22)17-18-9-10-19-5-1-2-6-20(19)15-18/h1-10,15,22,24H,11-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 609 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153141

(1-(1-Indan-2-yl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1Cc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-15-18-7-3-4-8-21(18)24(27)19-9-11-23(12-10-19)20-13-16-5-1-2-6-17(16)14-20/h1-8,19-20,22H,9-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153130

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(7.16,-4.52,;7.03,-3.01,;8.3,-2.12,;5.65,-2.33,;4.36,-3.23,;2.95,-2.57,;2.84,-1.01,;4.1,-.12,;5.52,-.8,;1.42,-.33,;.15,-1.22,;-1.23,-.54,;-1.36,.99,;-.09,1.86,;1.3,1.2,;-2.74,1.64,;-4.02,.83,;-4.3,-.68,;-5.73,-1.19,;-6.92,-.19,;-6.65,1.3,;-5.21,1.83,;-4.62,3.25,;-3.09,3.13,;-1.6,3.41,;-3.39,4.62,)| Show InChI InChI=1S/C20H31N3O2S/c1-15(2)16-7-9-17(10-8-16)22-13-11-18(12-14-22)23-20-6-4-3-5-19(20)21-26(23,24)25/h3-6,15-18,21H,7-14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 748 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153128

(1-(1-Cyclooctyl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show InChI InChI=1S/C20H31N3O2S/c24-26(25)21-16-17-8-6-7-11-20(17)23(26)19-12-14-22(15-13-19)18-9-4-2-1-3-5-10-18/h6-8,11,18-19,21H,1-5,9-10,12-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153139
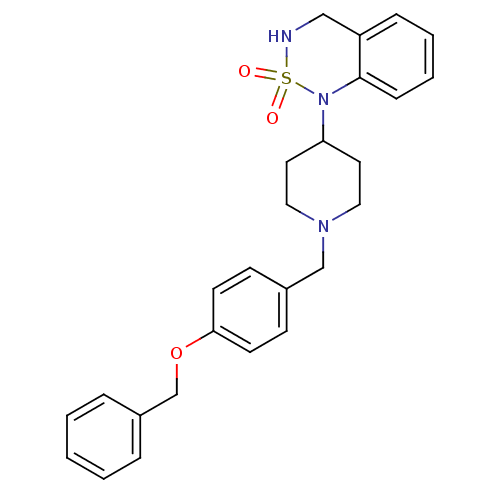
(1-[1-(4-Benzyloxy-benzyl)-piperidin-4-yl]-3,4-dihy...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc(OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C26H29N3O3S/c30-33(31)27-18-23-8-4-5-9-26(23)29(33)24-14-16-28(17-15-24)19-21-10-12-25(13-11-21)32-20-22-6-2-1-3-7-22/h1-13,24,27H,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153135

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153144

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-1,3-dih...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C22H23N3O2S/c26-28(27)23-21-7-3-4-8-22(21)25(28)20-11-13-24(14-12-20)16-17-9-10-18-5-1-2-6-19(18)15-17/h1-10,15,20,23H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153131

(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153139

(1-[1-(4-Benzyloxy-benzyl)-piperidin-4-yl]-3,4-dihy...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc(OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C26H29N3O3S/c30-33(31)27-18-23-8-4-5-9-26(23)29(33)24-14-16-28(17-15-24)19-21-10-12-25(13-11-21)32-20-22-6-2-1-3-7-22/h1-13,24,27H,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153134

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-3,4-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(9.9,-1.55,;8.44,-1.05,;7.29,-2.09,;5.82,-1.6,;4.68,-2.62,;3.22,-2.13,;2.92,-.62,;4.05,.4,;5.52,-.09,;1.46,-.14,;.31,-1.18,;-1.14,-.68,;-1.45,.82,;-.32,1.84,;1.15,1.35,;-2.91,1.28,;-3.89,.09,;-3.36,-1.34,;-4.31,-2.53,;-5.86,-2.27,;-6.39,-.85,;-5.41,.35,;-5.97,1.8,;-4.99,2.99,;-3.45,2.73,;-1.93,2.87,;-3.59,4.27,)| Show InChI InChI=1S/C21H33N3O2S/c1-2-5-17-8-10-19(11-9-17)23-14-12-20(13-15-23)24-21-7-4-3-6-18(21)16-22-27(24,25)26/h3-4,6-7,17,19-20,22H,2,5,8-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153141

(1-(1-Indan-2-yl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1Cc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-15-18-7-3-4-8-21(18)24(27)19-9-11-23(12-10-19)20-13-16-5-1-2-6-17(16)14-20/h1-8,19-20,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153146
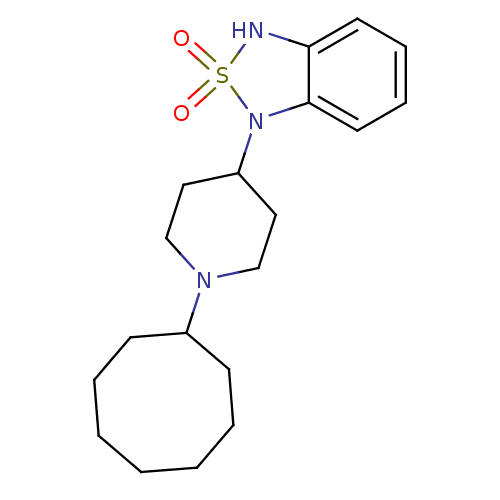
(1-(1-Cyclooctyl-piperidin-4-yl)-1,3-dihydro-benzo[...)Show InChI InChI=1S/C19H29N3O2S/c23-25(24)20-18-10-6-7-11-19(18)22(25)17-12-14-21(15-13-17)16-8-4-2-1-3-5-9-16/h6-7,10-11,16-17,20H,1-5,8-9,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153138
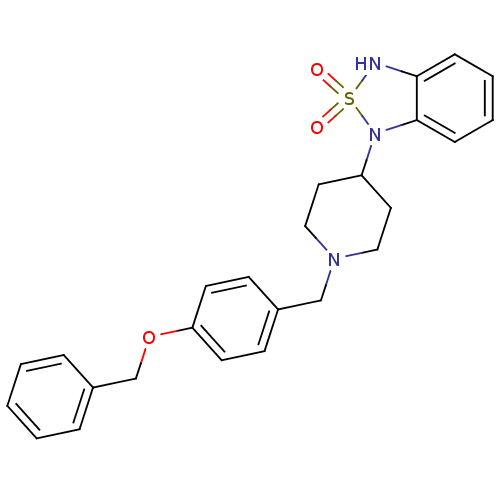
(1-[1-(4-Benzyloxy-benzyl)-piperidin-4-yl]-1,3-dihy...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc(OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C25H27N3O3S/c29-32(30)26-24-8-4-5-9-25(24)28(32)22-14-16-27(17-15-22)18-20-10-12-23(13-11-20)31-19-21-6-2-1-3-7-21/h1-13,22,26H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153132

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153143
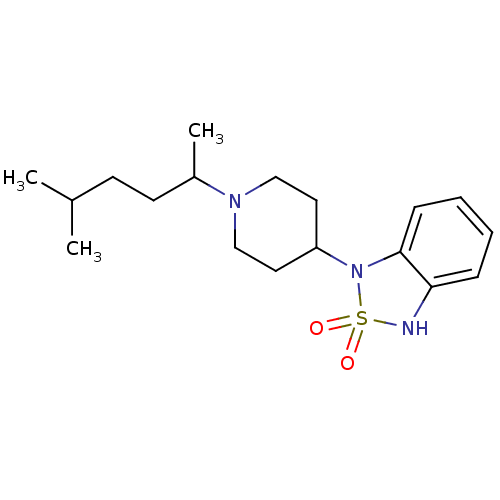
(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-1,3-dih...)Show InChI InChI=1S/C18H29N3O2S/c1-14(2)8-9-15(3)20-12-10-16(11-13-20)21-18-7-5-4-6-17(18)19-24(21,22)23/h4-7,14-16,19H,8-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153144

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-1,3-dih...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C22H23N3O2S/c26-28(27)23-21-7-3-4-8-22(21)25(28)20-11-13-24(14-12-20)16-17-9-10-18-5-1-2-6-19(18)15-17/h1-10,15,20,23H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153130

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(7.16,-4.52,;7.03,-3.01,;8.3,-2.12,;5.65,-2.33,;4.36,-3.23,;2.95,-2.57,;2.84,-1.01,;4.1,-.12,;5.52,-.8,;1.42,-.33,;.15,-1.22,;-1.23,-.54,;-1.36,.99,;-.09,1.86,;1.3,1.2,;-2.74,1.64,;-4.02,.83,;-4.3,-.68,;-5.73,-1.19,;-6.92,-.19,;-6.65,1.3,;-5.21,1.83,;-4.62,3.25,;-3.09,3.13,;-1.6,3.41,;-3.39,4.62,)| Show InChI InChI=1S/C20H31N3O2S/c1-15(2)16-7-9-17(10-8-16)22-13-11-18(12-14-22)23-20-6-4-3-5-19(20)21-26(23,24)25/h3-6,15-18,21H,7-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153145
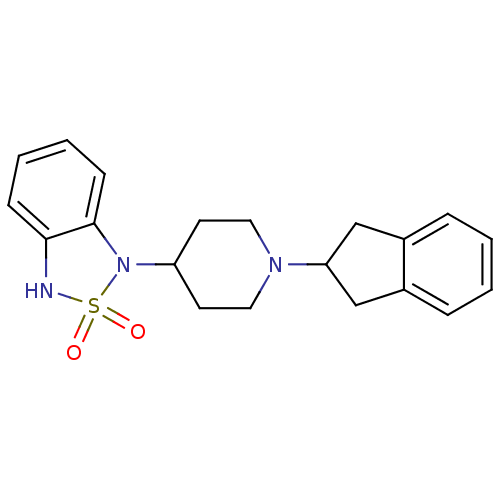
(1-(1-Indan-2-yl-piperidin-4-yl)-1,3-dihydro-benzo[...)Show InChI InChI=1S/C20H23N3O2S/c24-26(25)21-19-7-3-4-8-20(19)23(26)17-9-11-22(12-10-17)18-13-15-5-1-2-6-16(15)14-18/h1-8,17-18,21H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153132

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153131

(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153125

(CHEMBL364844 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES COC(=O)CN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.13,4.69,;4.78,5.41,;3.47,4.62,;3.51,3.08,;2.11,5.35,;.8,4.55,;-.55,5.3,;-1.84,4.48,;-3.19,5.23,;-4.5,4.43,;-4.46,2.89,;-3.12,2.14,;-1.82,2.94,;-.46,2.24,;-.44,.69,;-1.74,-.1,;-1.72,-1.67,;-.37,-2.38,;.94,-1.6,;.92,-.05,;-.37,-3.93,;-1.7,-4.71,;-1.7,-6.25,;-.37,-7.02,;.97,-6.25,;.97,-4.71,;-.34,-8.56,;-1.67,-9.35,;1.01,-9.33,;.85,3.03,;2.32,3.43,;1.25,1.53,)| Show InChI InChI=1S/C24H37N3O4S/c1-18(2)19-8-10-21(11-9-19)25-14-12-22(13-15-25)27-23-7-5-4-6-20(23)16-26(32(27,29)30)17-24(28)31-3/h4-7,18-19,21-22H,8-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153135

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153136
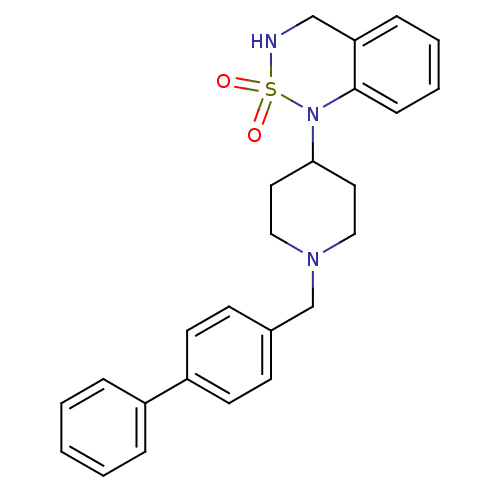
(1-(1-Biphenyl-4-ylmethyl-piperidin-4-yl)-3,4-dihyd...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C25H27N3O2S/c29-31(30)26-18-23-8-4-5-9-25(23)28(31)24-14-16-27(17-15-24)19-20-10-12-22(13-11-20)21-6-2-1-3-7-21/h1-13,24,26H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153127

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153145

(1-(1-Indan-2-yl-piperidin-4-yl)-1,3-dihydro-benzo[...)Show InChI InChI=1S/C20H23N3O2S/c24-26(25)21-19-7-3-4-8-20(19)23(26)17-9-11-22(12-10-17)18-13-15-5-1-2-6-16(15)14-18/h1-8,17-18,21H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153147
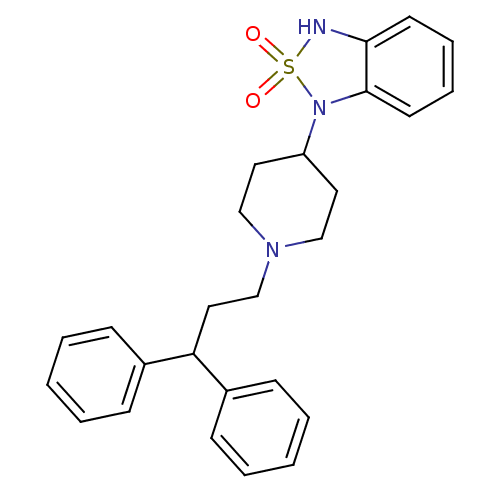
(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-1,3-dih...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C26H29N3O2S/c30-32(31)27-25-13-7-8-14-26(25)29(32)23-15-18-28(19-16-23)20-17-24(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-14,23-24,27H,15-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153126

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-1...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C21H31N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h3-4,7-8,16-19,22H,1-2,5-6,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153142
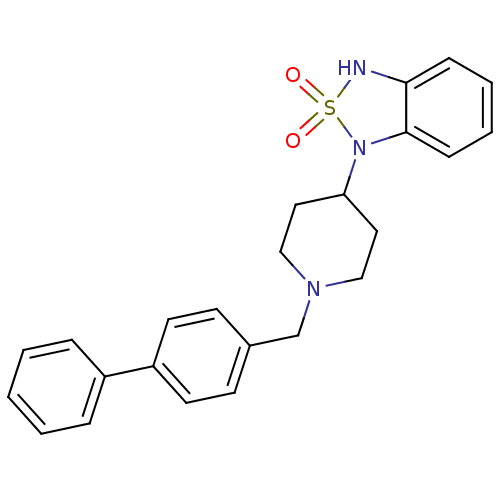
(1-(1-Biphenyl-4-ylmethyl-piperidin-4-yl)-1,3-dihyd...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C24H25N3O2S/c28-30(29)25-23-8-4-5-9-24(23)27(30)22-14-16-26(17-15-22)18-19-10-12-21(13-11-19)20-6-2-1-3-7-20/h1-13,22,25H,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153148

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-29(28)24-16-21-7-3-4-8-23(21)26(29)22-11-13-25(14-12-22)17-18-9-10-19-5-1-2-6-20(19)15-18/h1-10,15,22,24H,11-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153147

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-1,3-dih...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C26H29N3O2S/c30-32(31)27-25-13-7-8-14-26(25)29(32)23-15-18-28(19-16-23)20-17-24(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-14,23-24,27H,15-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153124

(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153148

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-29(28)24-16-21-7-3-4-8-23(21)26(29)22-11-13-25(14-12-22)17-18-9-10-19-5-1-2-6-20(19)15-18/h1-10,15,22,24H,11-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153141

(1-(1-Indan-2-yl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1Cc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-15-18-7-3-4-8-21(18)24(27)19-9-11-23(12-10-19)20-13-16-5-1-2-6-17(16)14-20/h1-8,19-20,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153146

(1-(1-Cyclooctyl-piperidin-4-yl)-1,3-dihydro-benzo[...)Show InChI InChI=1S/C19H29N3O2S/c23-25(24)20-18-10-6-7-11-19(18)22(25)17-12-14-21(15-13-17)16-8-4-2-1-3-5-9-16/h6-7,10-11,16-17,20H,1-5,8-9,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153133

(1-[1-(1,2,3,4-Tetrahydro-naphthalen-2-yl)-piperidi...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCc2ccccc2C1 Show InChI InChI=1S/C22H27N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h1-8,20-21,23H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153122

(CHEMBL181545 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(=O)OC(C)(C)C)S1(=O)=O |(-2.24,-9.73,;-.9,-8.94,;.44,-9.71,;-.93,-7.4,;-2.26,-6.62,;-2.26,-5.08,;-.93,-4.31,;.41,-5.08,;.41,-6.62,;-.94,-2.77,;-2.28,-2.04,;-2.31,-.48,;-1,.32,;.36,-.43,;.38,-1.97,;-1.02,1.86,;-2.38,2.56,;-3.69,1.77,;-5.02,2.52,;-5.07,4.06,;-3.76,4.86,;-2.4,4.11,;-1.11,4.9,;.24,4.18,;1.55,4.98,;2.91,4.25,;2.95,2.7,;4.22,5.04,;5.57,4.32,;4.85,2.96,;6.91,3.55,;6.34,5.65,;.29,2.66,;1.76,3.06,;.68,1.16,)| Show InChI InChI=1S/C27H43N3O4S/c1-20(2)21-10-12-23(13-11-21)28-16-14-24(15-17-28)30-25-9-7-6-8-22(25)18-29(35(30,32)33)19-26(31)34-27(3,4)5/h6-9,20-21,23-24H,10-19H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153133

(1-[1-(1,2,3,4-Tetrahydro-naphthalen-2-yl)-piperidi...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCc2ccccc2C1 Show InChI InChI=1S/C22H27N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h1-8,20-21,23H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153149
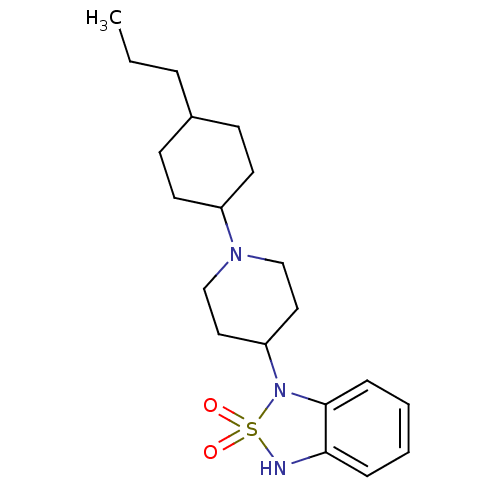
(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-1,3-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(9.58,-2.84,;8.2,-2.19,;6.93,-3.08,;5.55,-2.4,;4.26,-3.31,;2.86,-2.63,;2.74,-1.08,;4,-.17,;5.42,-.86,;1.32,-.4,;.05,-1.29,;-1.34,-.61,;-1.46,.92,;-.19,1.79,;1.21,1.13,;-2.84,1.57,;-4.13,.77,;-4.39,-.74,;-5.83,-1.26,;-7.01,-.26,;-6.74,1.23,;-5.3,1.76,;-4.73,3.18,;-3.18,3.06,;-1.69,3.34,;-3.48,4.55,)| Show InChI InChI=1S/C20H31N3O2S/c1-2-5-16-8-10-17(11-9-16)22-14-12-18(13-15-22)23-20-7-4-3-6-19(20)21-26(23,24)25/h3-4,6-7,16-18,21H,2,5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153123

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153139

(1-[1-(4-Benzyloxy-benzyl)-piperidin-4-yl]-3,4-dihy...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc(OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C26H29N3O3S/c30-33(31)27-18-23-8-4-5-9-26(23)29(33)24-14-16-28(17-15-24)19-21-10-12-25(13-11-21)32-20-22-6-2-1-3-7-22/h1-13,24,27H,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153143

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-1,3-dih...)Show InChI InChI=1S/C18H29N3O2S/c1-14(2)8-9-15(3)20-12-10-16(11-13-20)21-18-7-5-4-6-17(18)19-24(21,22)23/h4-7,14-16,19H,8-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153138

(1-[1-(4-Benzyloxy-benzyl)-piperidin-4-yl]-1,3-dihy...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc(OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C25H27N3O3S/c29-32(30)26-24-8-4-5-9-25(24)28(32)22-14-16-27(17-15-22)18-20-10-12-23(13-11-20)31-19-21-6-2-1-3-7-21/h1-13,22,26H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153126

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-1...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C21H31N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h3-4,7-8,16-19,22H,1-2,5-6,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153128

(1-(1-Cyclooctyl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show InChI InChI=1S/C20H31N3O2S/c24-26(25)21-16-17-8-6-7-11-20(17)23(26)19-12-14-22(15-13-19)18-9-4-2-1-3-5-10-18/h6-8,11,18-19,21H,1-5,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153130

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(7.16,-4.52,;7.03,-3.01,;8.3,-2.12,;5.65,-2.33,;4.36,-3.23,;2.95,-2.57,;2.84,-1.01,;4.1,-.12,;5.52,-.8,;1.42,-.33,;.15,-1.22,;-1.23,-.54,;-1.36,.99,;-.09,1.86,;1.3,1.2,;-2.74,1.64,;-4.02,.83,;-4.3,-.68,;-5.73,-1.19,;-6.92,-.19,;-6.65,1.3,;-5.21,1.83,;-4.62,3.25,;-3.09,3.13,;-1.6,3.41,;-3.39,4.62,)| Show InChI InChI=1S/C20H31N3O2S/c1-15(2)16-7-9-17(10-8-16)22-13-11-18(12-14-22)23-20-6-4-3-5-19(20)21-26(23,24)25/h3-6,15-18,21H,7-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153128

(1-(1-Cyclooctyl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show InChI InChI=1S/C20H31N3O2S/c24-26(25)21-16-17-8-6-7-11-20(17)23(26)19-12-14-22(15-13-19)18-9-4-2-1-3-5-10-18/h6-8,11,18-19,21H,1-5,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153136

(1-(1-Biphenyl-4-ylmethyl-piperidin-4-yl)-3,4-dihyd...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C25H27N3O2S/c29-31(30)26-18-23-8-4-5-9-25(23)28(31)24-14-16-27(17-15-24)19-20-10-12-22(13-11-20)21-6-2-1-3-7-21/h1-13,24,26H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153125

(CHEMBL364844 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES COC(=O)CN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.13,4.69,;4.78,5.41,;3.47,4.62,;3.51,3.08,;2.11,5.35,;.8,4.55,;-.55,5.3,;-1.84,4.48,;-3.19,5.23,;-4.5,4.43,;-4.46,2.89,;-3.12,2.14,;-1.82,2.94,;-.46,2.24,;-.44,.69,;-1.74,-.1,;-1.72,-1.67,;-.37,-2.38,;.94,-1.6,;.92,-.05,;-.37,-3.93,;-1.7,-4.71,;-1.7,-6.25,;-.37,-7.02,;.97,-6.25,;.97,-4.71,;-.34,-8.56,;-1.67,-9.35,;1.01,-9.33,;.85,3.03,;2.32,3.43,;1.25,1.53,)| Show InChI InChI=1S/C24H37N3O4S/c1-18(2)19-8-10-21(11-9-19)25-14-12-22(13-15-25)27-23-7-5-4-6-20(23)16-26(32(27,29)30)17-24(28)31-3/h4-7,18-19,21-22H,8-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153142

(1-(1-Biphenyl-4-ylmethyl-piperidin-4-yl)-1,3-dihyd...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C24H25N3O2S/c28-30(29)25-23-8-4-5-9-24(23)27(30)22-14-16-26(17-15-22)18-19-10-12-21(13-11-19)20-6-2-1-3-7-20/h1-13,22,25H,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153140
(1-[1-(1,2,3,4-Tetrahydro-naphthalen-2-yl)-piperidi...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h1-8,18-19,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153134

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-3,4-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(9.9,-1.55,;8.44,-1.05,;7.29,-2.09,;5.82,-1.6,;4.68,-2.62,;3.22,-2.13,;2.92,-.62,;4.05,.4,;5.52,-.09,;1.46,-.14,;.31,-1.18,;-1.14,-.68,;-1.45,.82,;-.32,1.84,;1.15,1.35,;-2.91,1.28,;-3.89,.09,;-3.36,-1.34,;-4.31,-2.53,;-5.86,-2.27,;-6.39,-.85,;-5.41,.35,;-5.97,1.8,;-4.99,2.99,;-3.45,2.73,;-1.93,2.87,;-3.59,4.27,)| Show InChI InChI=1S/C21H33N3O2S/c1-2-5-17-8-10-19(11-9-17)23-14-12-20(13-15-23)24-21-7-4-3-6-18(21)16-22-27(24,25)26/h3-4,6-7,17,19-20,22H,2,5,8-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153140

(1-[1-(1,2,3,4-Tetrahydro-naphthalen-2-yl)-piperidi...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h1-8,18-19,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153124

(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153127

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153123

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50153122

(CHEMBL181545 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(=O)OC(C)(C)C)S1(=O)=O |(-2.24,-9.73,;-.9,-8.94,;.44,-9.71,;-.93,-7.4,;-2.26,-6.62,;-2.26,-5.08,;-.93,-4.31,;.41,-5.08,;.41,-6.62,;-.94,-2.77,;-2.28,-2.04,;-2.31,-.48,;-1,.32,;.36,-.43,;.38,-1.97,;-1.02,1.86,;-2.38,2.56,;-3.69,1.77,;-5.02,2.52,;-5.07,4.06,;-3.76,4.86,;-2.4,4.11,;-1.11,4.9,;.24,4.18,;1.55,4.98,;2.91,4.25,;2.95,2.7,;4.22,5.04,;5.57,4.32,;4.85,2.96,;6.91,3.55,;6.34,5.65,;.29,2.66,;1.76,3.06,;.68,1.16,)| Show InChI InChI=1S/C27H43N3O4S/c1-20(2)21-10-12-23(13-11-21)28-16-14-24(15-17-28)30-25-9-7-6-8-22(25)18-29(35(30,32)33)19-26(31)34-27(3,4)5/h6-9,20-21,23-24H,10-19H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]U-69593 binding to human Opioid receptor kappa 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50153149

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-1,3-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(9.58,-2.84,;8.2,-2.19,;6.93,-3.08,;5.55,-2.4,;4.26,-3.31,;2.86,-2.63,;2.74,-1.08,;4,-.17,;5.42,-.86,;1.32,-.4,;.05,-1.29,;-1.34,-.61,;-1.46,.92,;-.19,1.79,;1.21,1.13,;-2.84,1.57,;-4.13,.77,;-4.39,-.74,;-5.83,-1.26,;-7.01,-.26,;-6.74,1.23,;-5.3,1.76,;-4.73,3.18,;-3.18,3.06,;-1.69,3.34,;-3.48,4.55,)| Show InChI InChI=1S/C20H31N3O2S/c1-2-5-16-8-10-17(11-9-16)22-14-12-18(13-15-22)23-20-7-4-3-6-19(20)21-26(23,24)25/h3-4,6-7,16-18,21H,2,5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]naltrindole binding to human Opioid receptor delta 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153146

(1-(1-Cyclooctyl-piperidin-4-yl)-1,3-dihydro-benzo[...)Show InChI InChI=1S/C19H29N3O2S/c23-25(24)20-18-10-6-7-11-19(18)22(25)17-12-14-21(15-13-17)16-8-4-2-1-3-5-9-16/h6-7,10-11,16-17,20H,1-5,8-9,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153133

(1-[1-(1,2,3,4-Tetrahydro-naphthalen-2-yl)-piperidi...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCc2ccccc2C1 Show InChI InChI=1S/C22H27N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h1-8,20-21,23H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153140

(1-[1-(1,2,3,4-Tetrahydro-naphthalen-2-yl)-piperidi...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h1-8,18-19,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153143

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-1,3-dih...)Show InChI InChI=1S/C18H29N3O2S/c1-14(2)8-9-15(3)20-12-10-16(11-13-20)21-18-7-5-4-6-17(18)19-24(21,22)23/h4-7,14-16,19H,8-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153147

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-1,3-dih...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C26H29N3O2S/c30-32(31)27-25-13-7-8-14-26(25)29(32)23-15-18-28(19-16-23)20-17-24(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-14,23-24,27H,15-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153149

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-1,3-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(9.58,-2.84,;8.2,-2.19,;6.93,-3.08,;5.55,-2.4,;4.26,-3.31,;2.86,-2.63,;2.74,-1.08,;4,-.17,;5.42,-.86,;1.32,-.4,;.05,-1.29,;-1.34,-.61,;-1.46,.92,;-.19,1.79,;1.21,1.13,;-2.84,1.57,;-4.13,.77,;-4.39,-.74,;-5.83,-1.26,;-7.01,-.26,;-6.74,1.23,;-5.3,1.76,;-4.73,3.18,;-3.18,3.06,;-1.69,3.34,;-3.48,4.55,)| Show InChI InChI=1S/C20H31N3O2S/c1-2-5-16-8-10-17(11-9-16)22-14-12-18(13-15-22)23-20-7-4-3-6-19(20)21-26(23,24)25/h3-4,6-7,16-18,21H,2,5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153126

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-1...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C21H31N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h3-4,7-8,16-19,22H,1-2,5-6,9-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153145

(1-(1-Indan-2-yl-piperidin-4-yl)-1,3-dihydro-benzo[...)Show InChI InChI=1S/C20H23N3O2S/c24-26(25)21-19-7-3-4-8-20(19)23(26)17-9-11-22(12-10-17)18-13-15-5-1-2-6-16(15)14-18/h1-8,17-18,21H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153138

(1-[1-(4-Benzyloxy-benzyl)-piperidin-4-yl]-1,3-dihy...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc(OCc3ccccc3)cc2)CC1 Show InChI InChI=1S/C25H27N3O3S/c29-32(30)26-24-8-4-5-9-25(24)28(32)22-14-16-27(17-15-22)18-20-10-12-23(13-11-20)31-19-21-6-2-1-3-7-21/h1-13,22,26H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153136

(1-(1-Biphenyl-4-ylmethyl-piperidin-4-yl)-3,4-dihyd...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C25H27N3O2S/c29-31(30)26-18-23-8-4-5-9-25(23)28(31)24-14-16-27(17-15-24)19-20-10-12-22(13-11-20)21-6-2-1-3-7-21/h1-13,24,26H,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153142

(1-(1-Biphenyl-4-ylmethyl-piperidin-4-yl)-1,3-dihyd...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C24H25N3O2S/c28-30(29)25-23-8-4-5-9-24(23)27(30)22-14-16-26(17-15-22)18-19-10-12-21(13-11-19)20-6-2-1-3-7-20/h1-13,22,25H,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153144

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-1,3-dih...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C22H23N3O2S/c26-28(27)23-21-7-3-4-8-22(21)25(28)20-11-13-24(14-12-20)16-17-9-10-18-5-1-2-6-19(18)15-17/h1-10,15,20,23H,11-14,16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 799 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153148

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-29(28)24-16-21-7-3-4-8-23(21)26(29)22-11-13-25(14-12-22)17-18-9-10-19-5-1-2-6-20(19)15-18/h1-10,15,22,24H,11-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.59E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153127

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 555 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153126

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-1...)Show SMILES O=S1(=O)Nc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C21H31N3O2S/c25-27(26)22-20-7-3-4-8-21(20)24(27)18-11-13-23(14-12-18)19-10-9-16-5-1-2-6-17(16)15-19/h3-4,7-8,16-19,22H,1-2,5-6,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153125

(CHEMBL364844 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES COC(=O)CN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.13,4.69,;4.78,5.41,;3.47,4.62,;3.51,3.08,;2.11,5.35,;.8,4.55,;-.55,5.3,;-1.84,4.48,;-3.19,5.23,;-4.5,4.43,;-4.46,2.89,;-3.12,2.14,;-1.82,2.94,;-.46,2.24,;-.44,.69,;-1.74,-.1,;-1.72,-1.67,;-.37,-2.38,;.94,-1.6,;.92,-.05,;-.37,-3.93,;-1.7,-4.71,;-1.7,-6.25,;-.37,-7.02,;.97,-6.25,;.97,-4.71,;-.34,-8.56,;-1.67,-9.35,;1.01,-9.33,;.85,3.03,;2.32,3.43,;1.25,1.53,)| Show InChI InChI=1S/C24H37N3O4S/c1-18(2)19-8-10-21(11-9-19)25-14-12-22(13-15-25)27-23-7-5-4-6-20(23)16-26(32(27,29)30)17-24(28)31-3/h4-7,18-19,21-22H,8-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153127

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153131

(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153123

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153130

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2NS1(=O)=O |(7.16,-4.52,;7.03,-3.01,;8.3,-2.12,;5.65,-2.33,;4.36,-3.23,;2.95,-2.57,;2.84,-1.01,;4.1,-.12,;5.52,-.8,;1.42,-.33,;.15,-1.22,;-1.23,-.54,;-1.36,.99,;-.09,1.86,;1.3,1.2,;-2.74,1.64,;-4.02,.83,;-4.3,-.68,;-5.73,-1.19,;-6.92,-.19,;-6.65,1.3,;-5.21,1.83,;-4.62,3.25,;-3.09,3.13,;-1.6,3.41,;-3.39,4.62,)| Show InChI InChI=1S/C20H31N3O2S/c1-15(2)16-7-9-17(10-8-16)22-13-11-18(12-14-22)23-20-6-4-3-5-19(20)21-26(23,24)25/h3-6,15-18,21H,7-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153128

(1-(1-Cyclooctyl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show InChI InChI=1S/C20H31N3O2S/c24-26(25)21-16-17-8-6-7-11-20(17)23(26)19-12-14-22(15-13-19)18-9-4-2-1-3-5-10-18/h6-8,11,18-19,21H,1-5,9-10,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 202 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153148

(1-(1-Naphthalen-2-ylmethyl-piperidin-4-yl)-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(Cc2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-29(28)24-16-21-7-3-4-8-23(21)26(29)22-11-13-25(14-12-22)17-18-9-10-19-5-1-2-6-20(19)15-18/h1-10,15,22,24H,11-14,16-17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153137

(3-Butyl-1-[1-(4-isopropyl-cyclohexyl)-piperidin-4-...)Show SMILES CCCCN1Cc2ccccc2N(C2CCN(CC2)C2CCC(CC2)C(C)C)S1(=O)=O |(6.25,4.77,;4.89,5.53,;3.59,4.72,;2.23,5.45,;.92,4.65,;-.44,5.38,;-1.72,4.6,;-3.08,5.33,;-4.39,4.54,;-4.34,2.99,;-3.01,2.24,;-1.7,3.04,;-.34,2.34,;-.32,.8,;-1.63,,;-1.61,-1.57,;-.25,-2.29,;1.06,-1.5,;1.04,.05,;-.25,-3.83,;1.08,-4.61,;1.08,-6.15,;-.25,-6.92,;-1.58,-6.15,;-1.58,-4.61,;-.23,-8.46,;1.13,-9.24,;-1.56,-9.26,;.97,3.13,;2.44,3.55,;1.36,1.64,)| Show InChI InChI=1S/C25H41N3O2S/c1-4-5-16-27-19-22-8-6-7-9-25(22)28(31(27,29)30)24-14-17-26(18-15-24)23-12-10-21(11-13-23)20(2)3/h6-9,20-21,23-24H,4-5,10-19H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153132

(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153141

(1-(1-Indan-2-yl-piperidin-4-yl)-3,4-dihydro-1H-ben...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1Cc2ccccc2C1 Show InChI InChI=1S/C21H25N3O2S/c25-27(26)22-15-18-7-3-4-8-21(18)24(27)19-9-11-23(12-10-19)20-13-16-5-1-2-6-17(16)14-20/h1-8,19-20,22H,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 496 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153135

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 801 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153122

(CHEMBL181545 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(=O)OC(C)(C)C)S1(=O)=O |(-2.24,-9.73,;-.9,-8.94,;.44,-9.71,;-.93,-7.4,;-2.26,-6.62,;-2.26,-5.08,;-.93,-4.31,;.41,-5.08,;.41,-6.62,;-.94,-2.77,;-2.28,-2.04,;-2.31,-.48,;-1,.32,;.36,-.43,;.38,-1.97,;-1.02,1.86,;-2.38,2.56,;-3.69,1.77,;-5.02,2.52,;-5.07,4.06,;-3.76,4.86,;-2.4,4.11,;-1.11,4.9,;.24,4.18,;1.55,4.98,;2.91,4.25,;2.95,2.7,;4.22,5.04,;5.57,4.32,;4.85,2.96,;6.91,3.55,;6.34,5.65,;.29,2.66,;1.76,3.06,;.68,1.16,)| Show InChI InChI=1S/C27H43N3O4S/c1-20(2)21-10-12-23(13-11-21)28-16-14-24(15-17-28)30-25-9-7-6-8-22(25)18-29(35(30,32)33)19-26(31)34-27(3,4)5/h6-9,20-21,23-24H,10-19H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 484 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153124

(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153129

(1-[1-(Decahydro-naphthalen-2-yl)-piperidin-4-yl]-3...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CC1)C1CCC2CCCCC2C1 Show InChI InChI=1S/C22H33N3O2S/c26-28(27)23-16-19-7-3-4-8-22(19)25(28)20-11-13-24(14-12-20)21-10-9-17-5-1-2-6-18(17)15-21/h3-4,7-8,17-18,20-21,23H,1-2,5-6,9-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153123

(1-[1-(1,4-Dimethyl-pentyl)-piperidin-4-yl]-3,4-dih...)Show InChI InChI=1S/C19H31N3O2S/c1-15(2)8-9-16(3)21-12-10-18(11-13-21)22-19-7-5-4-6-17(19)14-20-25(22,23)24/h4-7,15-16,18,20H,8-14H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153131

(CHEMBL185415 | {1-[1-(4-Isopropyl-cyclohexyl)-pipe...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC#N)S1(=O)=O |(1.34,-9.03,;-.02,-8.25,;-1.35,-9.05,;-.04,-6.71,;-1.37,-5.95,;-1.37,-4.4,;-.04,-3.62,;1.29,-4.4,;1.29,-5.95,;-.04,-2.07,;-1.39,-1.36,;-1.43,.21,;-.11,1.01,;1.25,.27,;1.27,-1.29,;-.14,2.55,;-1.49,3.27,;-2.8,2.46,;-4.13,3.2,;-4.18,4.75,;-2.87,5.54,;-1.51,4.79,;-.23,5.6,;1.13,4.86,;2.44,5.67,;3.8,4.95,;5.14,4.19,;1.17,3.34,;2.65,3.74,;1.57,1.85,)| Show InChI InChI=1S/C23H34N4O2S/c1-18(2)19-7-9-21(10-8-19)25-14-11-22(12-15-25)27-23-6-4-3-5-20(23)17-26(16-13-24)30(27,28)29/h3-6,18-19,21-22H,7-12,14-17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 344 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153134

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-3,4-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(9.9,-1.55,;8.44,-1.05,;7.29,-2.09,;5.82,-1.6,;4.68,-2.62,;3.22,-2.13,;2.92,-.62,;4.05,.4,;5.52,-.09,;1.46,-.14,;.31,-1.18,;-1.14,-.68,;-1.45,.82,;-.32,1.84,;1.15,1.35,;-2.91,1.28,;-3.89,.09,;-3.36,-1.34,;-4.31,-2.53,;-5.86,-2.27,;-6.39,-.85,;-5.41,.35,;-5.97,1.8,;-4.99,2.99,;-3.45,2.73,;-1.93,2.87,;-3.59,4.27,)| Show InChI InChI=1S/C21H33N3O2S/c1-2-5-17-8-10-19(11-9-17)23-14-12-20(13-15-23)24-21-7-4-3-6-18(21)16-22-27(24,25)26/h3-4,6-7,17,19-20,22H,2,5,8-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153135

(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153134

(1-[1-(4-Propyl-cyclohexyl)-piperidin-4-yl]-3,4-dih...)Show SMILES CCCC1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(9.9,-1.55,;8.44,-1.05,;7.29,-2.09,;5.82,-1.6,;4.68,-2.62,;3.22,-2.13,;2.92,-.62,;4.05,.4,;5.52,-.09,;1.46,-.14,;.31,-1.18,;-1.14,-.68,;-1.45,.82,;-.32,1.84,;1.15,1.35,;-2.91,1.28,;-3.89,.09,;-3.36,-1.34,;-4.31,-2.53,;-5.86,-2.27,;-6.39,-.85,;-5.41,.35,;-5.97,1.8,;-4.99,2.99,;-3.45,2.73,;-1.93,2.87,;-3.59,4.27,)| Show InChI InChI=1S/C21H33N3O2S/c1-2-5-17-8-10-19(11-9-17)23-14-12-20(13-15-23)24-21-7-4-3-6-18(21)16-22-27(24,25)26/h3-4,6-7,17,19-20,22H,2,5,8-16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153121

(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data