

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor in guinea pig caudate assessed as inhibition of U69593-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50350976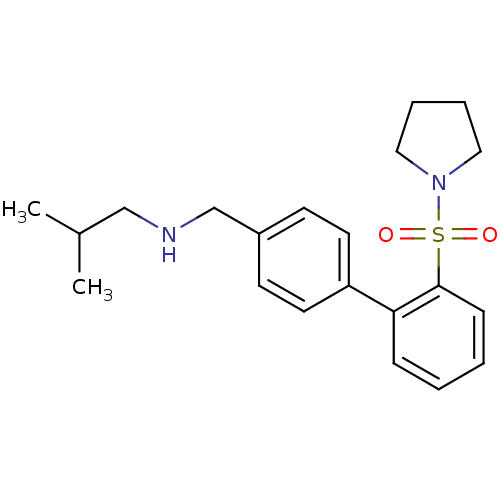 (CHEMBL1818341) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of U69593-stimulated [35S]-GTP[gammaS] bindin... | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012152 (CHEMBL3264441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from kappa opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at mu opioid receptor in guinea pig caudate assessed as inhibition of DAMGO-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012151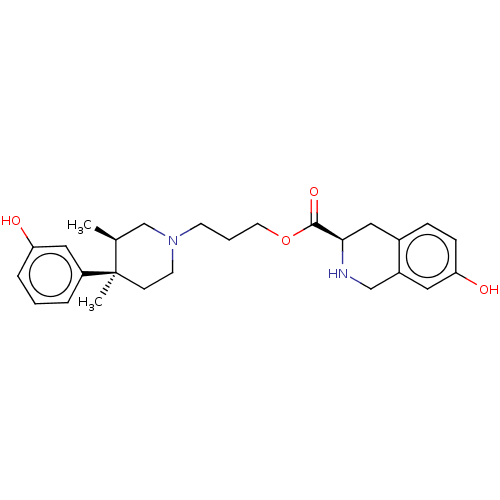 (CHEMBL3264440) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mu opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50350976 (CHEMBL1818341) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012151 (CHEMBL3264440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from kappa opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012152 (CHEMBL3264441) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mu opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012153 (zyklophin) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from kappa opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012150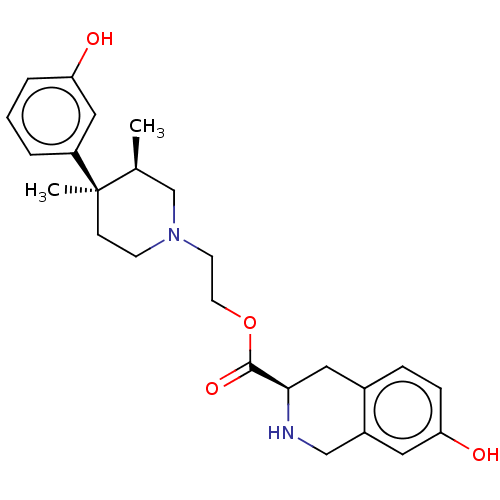 (CHEMBL3264439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from kappa opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012150 (CHEMBL3264439) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from mu opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012151 (CHEMBL3264440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from delta opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012150 (CHEMBL3264439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 475 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from delta opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM54817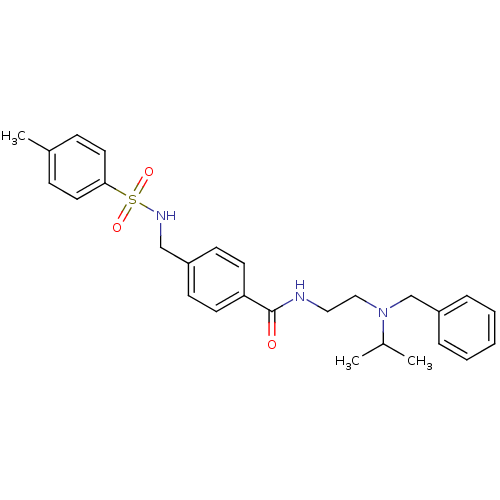 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM71610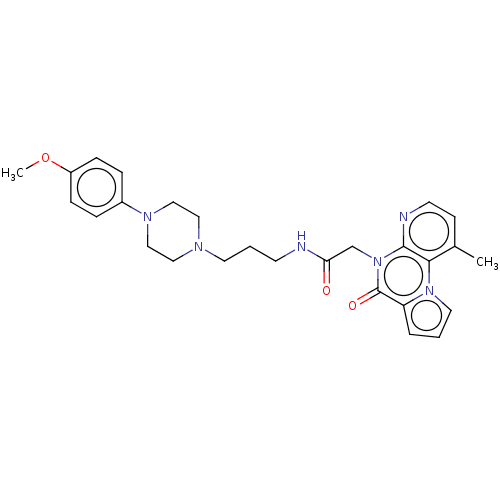 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to dopamine D3 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM75566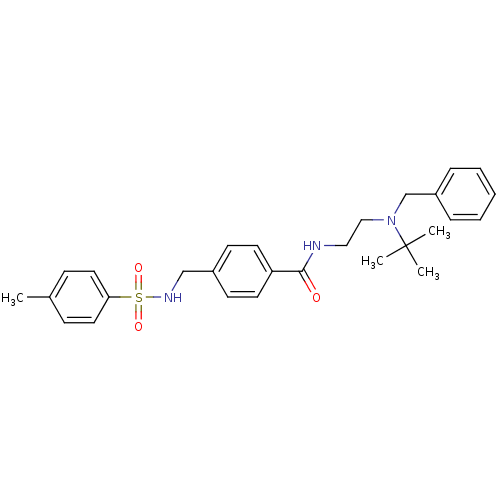 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M4 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM71610 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to histamine H1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to NET (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to sigma-1 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M4 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM71610 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to NET (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to adrenergic alpha2C receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Binding affinity to 5-HT2B receptor (unknown origin) | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012152 (CHEMBL3264441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from delta opioid receptor (unknown origin) expressed in CHO cells membranes | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71610 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71613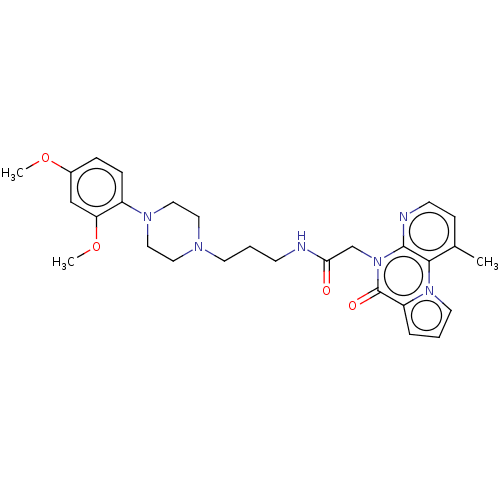 (KSC-1-265 | KUC104505N | cid_44665679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012149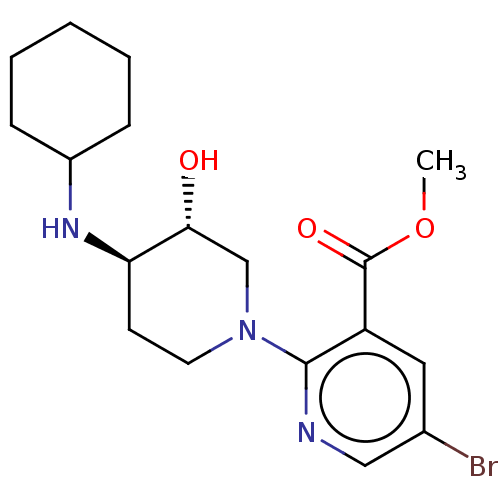 (CHEMBL3264446 | US10118915, Compound 105 | US96829...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of U69593-stimulated [35S]-GTP[gammaS] bindin... | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71611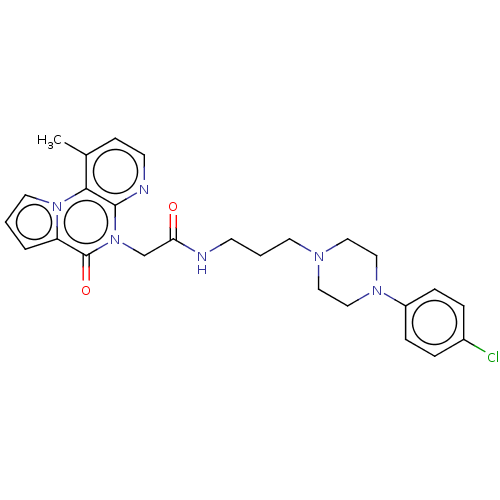 (KSC-1-261 | KUC104503N | cid_44665687) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71610 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71613 (KSC-1-265 | KUC104505N | cid_44665679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012149 (CHEMBL3264446 | US10118915, Compound 105 | US96829...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012148 (CHEMBL3183148) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned kappa opioid receptor expressed in CHO cells assessed as inhibition of U69593-stimulated [35S]-GTP[gammaS] bindin... | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71581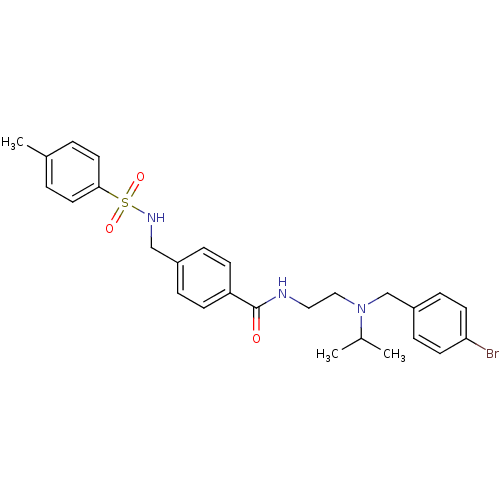 (KSC-5-280C | KUC104162N | N-[2-[(4-bromobenzyl)-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71581 (KSC-5-280C | KUC104162N | N-[2-[(4-bromobenzyl)-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71611 (KSC-1-261 | KUC104503N | cid_44665687) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71619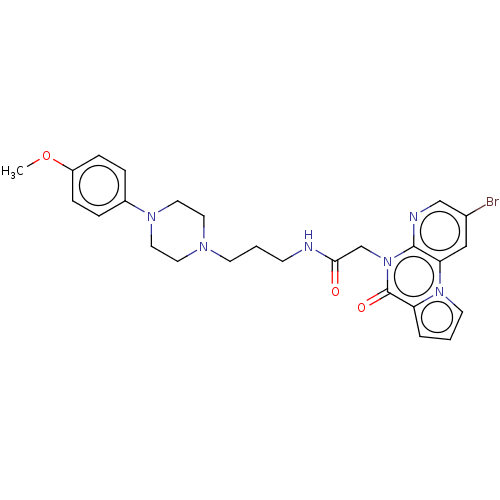 (KSC-1-278 | KUC104646N | cid_45100475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65828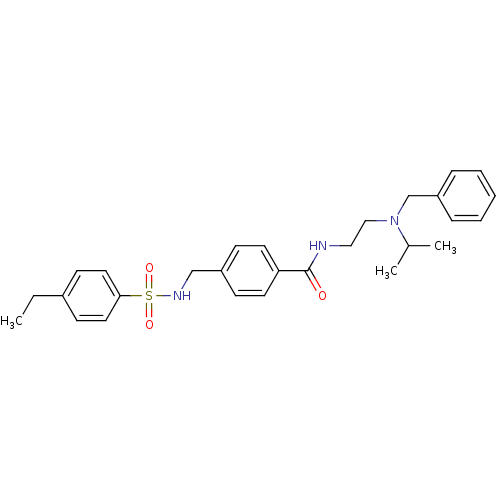 (4-[[(4-ethylphenyl)sulfonylamino]methyl]-N-[2-[(ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM54842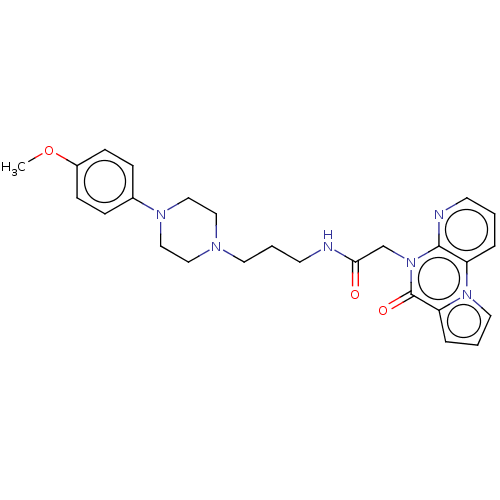 (MLS001123392 | SMR000627745 | cid_22553442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM54842 (MLS001123392 | SMR000627745 | cid_22553442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50350976 (CHEMBL1818341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of human ERG channel | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65828 (4-[[(4-ethylphenyl)sulfonylamino]methyl]-N-[2-[(ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71567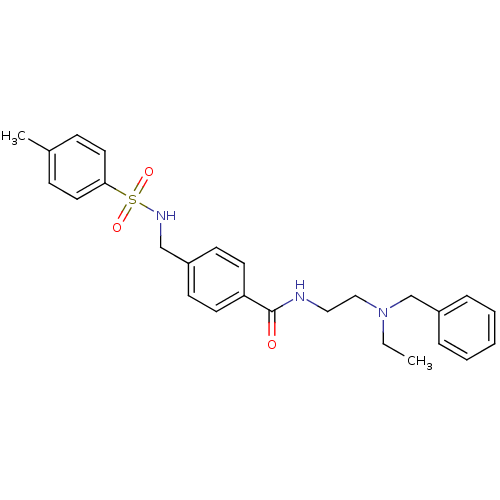 (KSC-11-23-1 | KUC104147N | N-[2-[benzyl(ethyl)amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71619 (KSC-1-278 | KUC104646N | cid_45100475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012149 (CHEMBL3264446 | US10118915, Compound 105 | US96829...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned delta opioid receptor expressed in CHO cells assessed as inhibition of SNC-80-stimulated [35S]-GTP[gammaS] bindin... | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM62203 (2-(4-ketopyrrolo[1,2-a]quinoxalin-5-yl)-N-[3-[4-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM71567 (KSC-11-23-1 | KUC104147N | N-[2-[benzyl(ethyl)amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012148 (CHEMBL3183148) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012148 (CHEMBL3183148) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned delta opioid receptor expressed in CHO cells assessed as inhibition of SNC-80-stimulated [35S]-GTP[gammaS] bindin... | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM71567 (KSC-11-23-1 | KUC104147N | N-[2-[benzyl(ethyl)amin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM71619 (KSC-1-278 | KUC104646N | cid_45100475) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM62203 (2-(4-ketopyrrolo[1,2-a]quinoxalin-5-yl)-N-[3-[4-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65830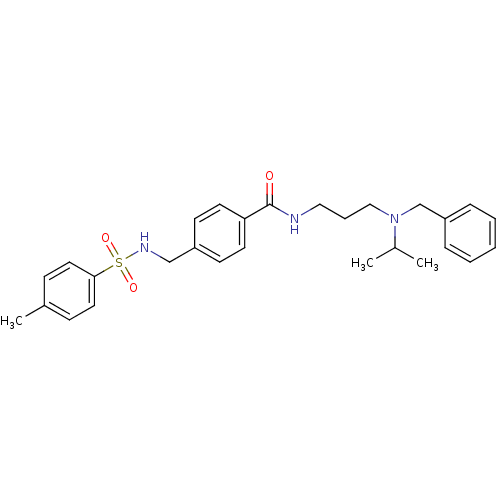 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[3-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by DiscoveryRx b-arrestin PathHunter assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM71613 (KSC-1-265 | KUC104505N | cid_44665679) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM71581 (KSC-5-280C | KUC104162N | N-[2-[(4-bromobenzyl)-is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM65828 (4-[[(4-ethylphenyl)sulfonylamino]methyl]-N-[2-[(ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM75566 (KSC-11-114-1 | KUC105392N | N-[2-[benzyl(tert-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM71567 (KSC-11-23-1 | KUC104147N | N-[2-[benzyl(ethyl)amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM54817 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[2-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM54842 (MLS001123392 | SMR000627745 | cid_22553442) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM71611 (KSC-1-261 | KUC104503N | cid_44665687) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM71619 (KSC-1-278 | KUC104646N | cid_45100475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM62203 (2-(4-ketopyrrolo[1,2-a]quinoxalin-5-yl)-N-[3-[4-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM71611 (KSC-1-261 | KUC104503N | cid_44665687) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM71613 (KSC-1-265 | KUC104505N | cid_44665679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM71610 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM65830 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[3-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM65830 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[3-[(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of kappa opioid receptor (unknown origin) using dynorphin A by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM54842 (MLS001123392 | SMR000627745 | cid_22553442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM65830 (4-[[(4-methylphenyl)sulfonylamino]methyl]-N-[3-[(p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM71581 (KSC-5-280C | KUC104162N | N-[2-[(4-bromobenzyl)-is...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM62203 (2-(4-ketopyrrolo[1,2-a]quinoxalin-5-yl)-N-[3-[4-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM71610 (KSC-1-256 | KUC104502N | ML190 | cid_44665680) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) using DAMGO by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM65828 (4-[[(4-ethylphenyl)sulfonylamino]methyl]-N-[2-[(ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of delta opioid receptor (unknown origin) using SNC-80 by high content imaging beta-arrestin translocation assay | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50154200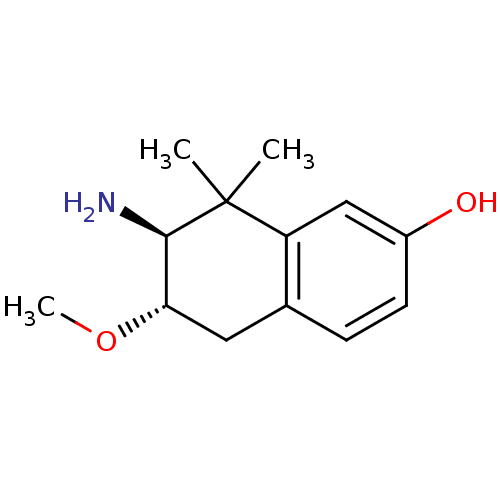 (7-amino-6-methoxy-8,8-dimethyl-5,6,7,8-tetrahydron...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 234 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50012155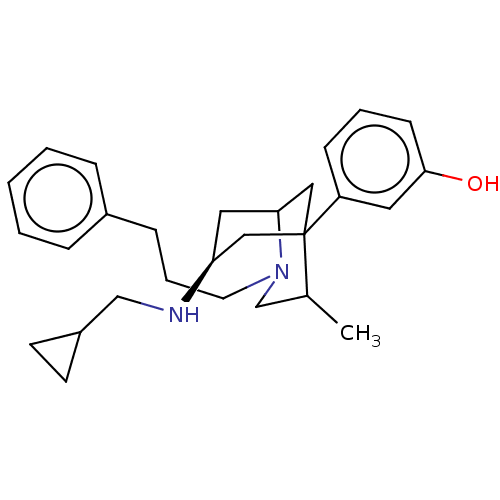 (CHEMBL3264442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50116621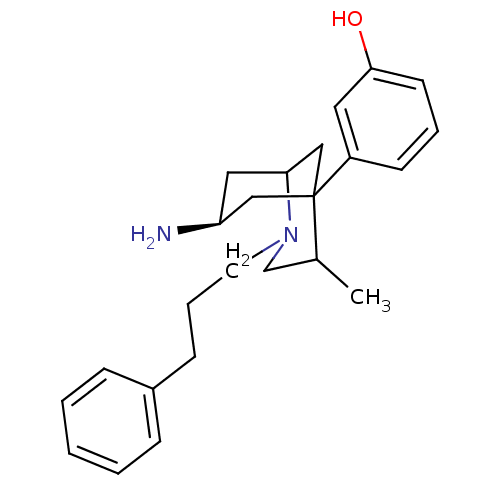 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012155 (CHEMBL3264442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50012155 (CHEMBL3264442) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50116621 (3-[7-Amino-4-methyl-2-(3-phenyl-propyl)-2-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human cloned delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||