 Found 55 hits with Last Name = 'launchbury' and Initial = 'sb'
Found 55 hits with Last Name = 'launchbury' and Initial = 'sb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121445
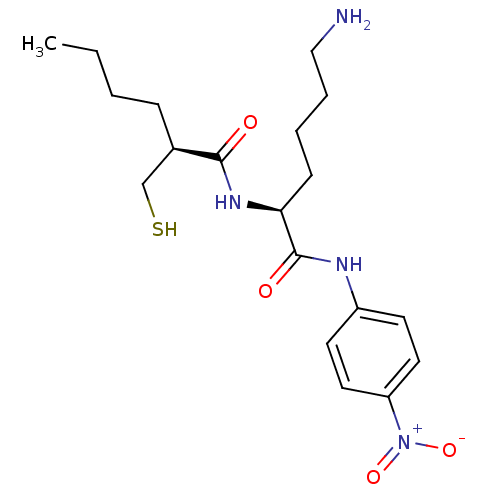
((S)-2-Mercaptomethyl-hexanoic acid [(S)-5-amino-1-...)Show SMILES CCCC[C@H](CS)C(=O)N[C@@H](CCCCN)C(=O)Nc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C19H30N4O4S/c1-2-3-6-14(13-28)18(24)22-17(7-4-5-12-20)19(25)21-15-8-10-16(11-9-15)23(26)27/h8-11,14,17,28H,2-7,12-13,20H2,1H3,(H,21,25)(H,22,24)/t14-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Fe of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121442
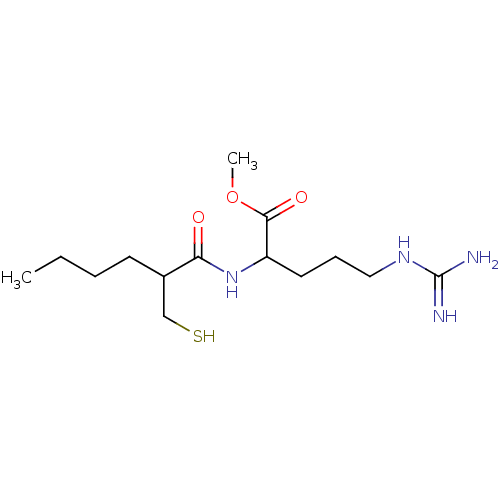
(5-Guanidino-2-(2-mercaptomethyl-hexanoylamino)-pen...)Show InChI InChI=1S/C14H28N4O3S/c1-3-4-6-10(9-22)12(19)18-11(13(20)21-2)7-5-8-17-14(15)16/h10-11,22H,3-9H2,1-2H3,(H,18,19)(H4,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121444
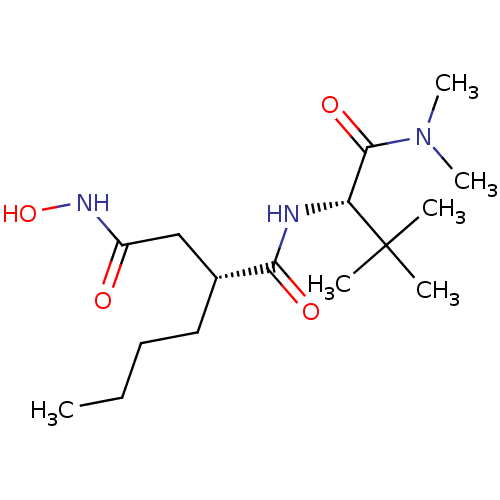
((R)-2-Butyl-N*1*-((S)-1-dimethylcarbamoyl-2,2-dime...)Show SMILES CCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-11(10-12(20)18-23)14(21)17-13(16(2,3)4)15(22)19(5)6/h11,13,23H,7-10H2,1-6H3,(H,17,21)(H,18,20)/t11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131303
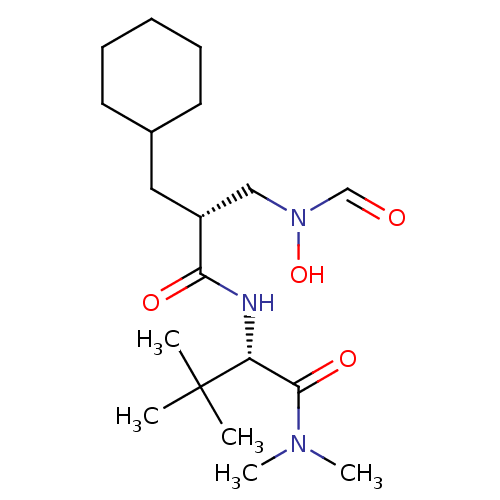
((S)-2-[(R)-2-Cyclohexylmethyl-3-(formyl-hydroxy-am...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@H](CC1CCCCC1)CN(O)C=O)C(C)(C)C Show InChI InChI=1S/C19H35N3O4/c1-19(2,3)16(18(25)21(4)5)20-17(24)15(12-22(26)13-23)11-14-9-7-6-8-10-14/h13-16,26H,6-12H2,1-5H3,(H,20,24)/t15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Antibacterial activity of the compound against E. coli Peptide deformylase. Ni |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131290
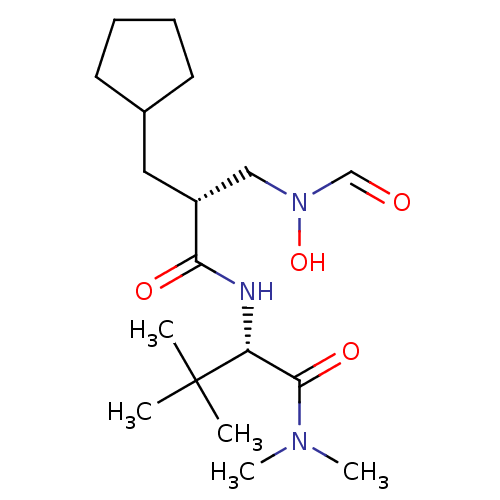
((S)-2-[(R)-2-Cyclopentylmethyl-3-(formyl-hydroxy-a...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@H](CC1CCCC1)CN(O)C=O)C(C)(C)C Show InChI InChI=1S/C18H33N3O4/c1-18(2,3)15(17(24)20(4)5)19-16(23)14(11-21(25)12-22)10-13-8-6-7-9-13/h12-15,25H,6-11H2,1-5H3,(H,19,23)/t14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Antibacterial activity of the compound against E. coli Peptide deformylase. Ni |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131292
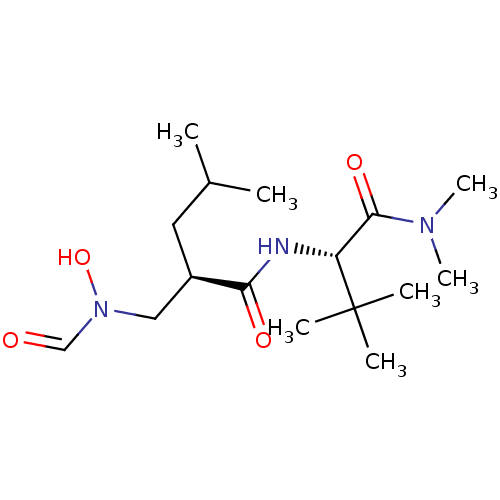
((R)-2-[(Formyl-hydroxy-amino)-methyl]-4-methyl-pen...)Show SMILES CC(C)C[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-11(2)8-12(9-19(23)10-20)14(21)17-13(16(3,4)5)15(22)18(6)7/h10-13,23H,8-9H2,1-7H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131304
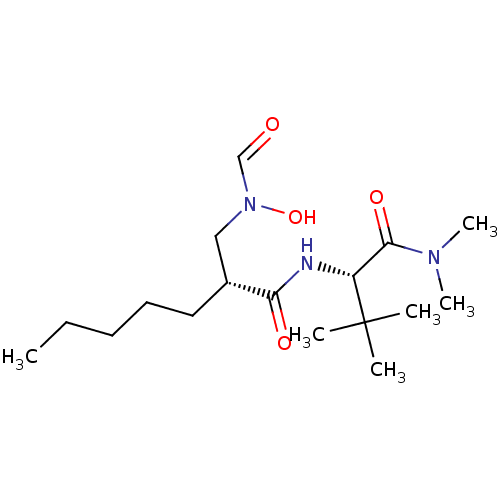
((R)-2-[(Formyl-hydroxy-amino)-methyl]-heptanoic ac...)Show SMILES CCCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C17H33N3O4/c1-7-8-9-10-13(11-20(24)12-21)15(22)18-14(17(2,3)4)16(23)19(5)6/h12-14,24H,7-11H2,1-6H3,(H,18,22)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131301
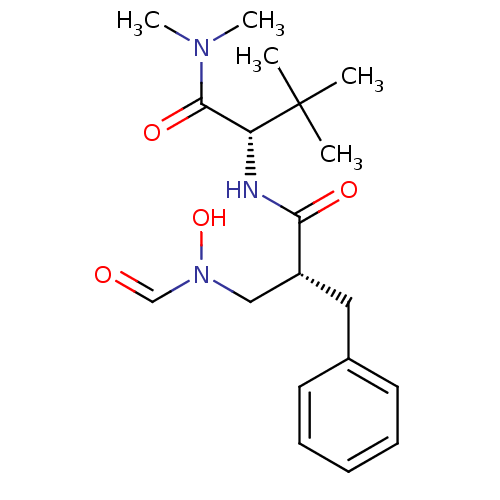
((S)-2-[(R)-2-Benzyl-3-(formyl-hydroxy-amino)-propi...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@@H](CN(O)C=O)Cc1ccccc1)C(C)(C)C Show InChI InChI=1S/C19H29N3O4/c1-19(2,3)16(18(25)21(4)5)20-17(24)15(12-22(26)13-23)11-14-9-7-6-8-10-14/h6-10,13,15-16,26H,11-12H2,1-5H3,(H,20,24)/t15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131288
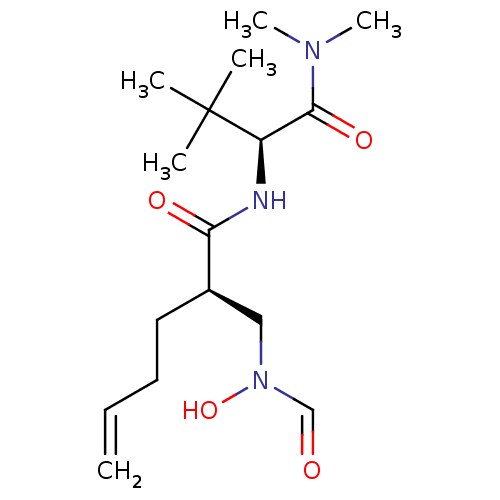
((R)-2-[(Formyl-hydroxy-amino)-methyl]-hex-5-enoic ...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@H](CCC=C)CN(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H29N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h7,11-13,23H,1,8-10H2,2-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131302
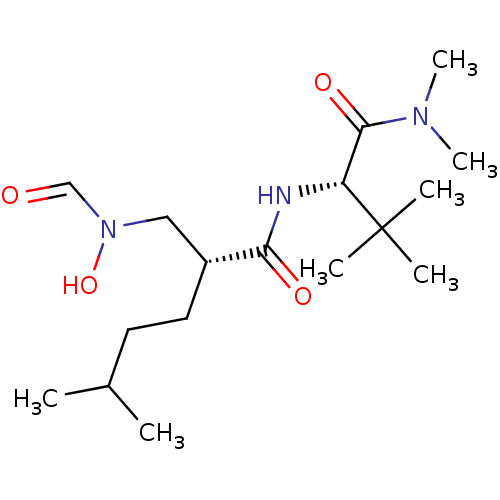
((R)-2-[(Formyl-hydroxy-amino)-methyl]-5-methyl-hex...)Show SMILES CC(C)CC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C17H33N3O4/c1-12(2)8-9-13(10-20(24)11-21)15(22)18-14(17(3,4)5)16(23)19(6)7/h11-14,24H,8-10H2,1-7H3,(H,18,22)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131307
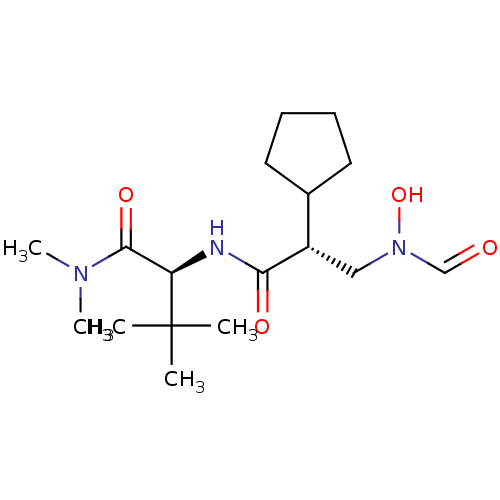
((S)-2-[(R)-2-Cyclopentyl-3-(formyl-hydroxy-amino)-...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@@H](CN(O)C=O)C1CCCC1)C(C)(C)C Show InChI InChI=1S/C17H31N3O4/c1-17(2,3)14(16(23)19(4)5)18-15(22)13(10-20(24)11-21)12-8-6-7-9-12/h11-14,24H,6-10H2,1-5H3,(H,18,22)/t13-,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131295
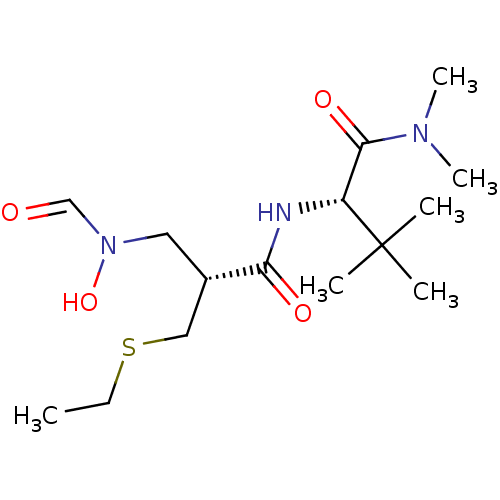
((S)-2-[(R)-2-Ethylsulfanylmethyl-3-(formyl-hydroxy...)Show SMILES CCSC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C15H29N3O4S/c1-7-23-9-11(8-18(22)10-19)13(20)16-12(15(2,3)4)14(21)17(5)6/h10-12,22H,7-9H2,1-6H3,(H,16,20)/t11-,12+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131291
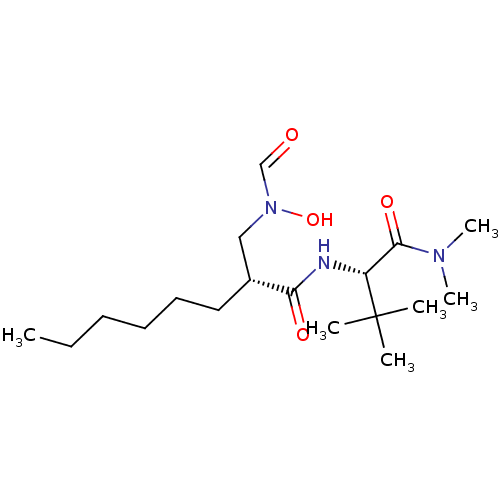
((R)-2-[(Formyl-hydroxy-amino)-methyl]-octanoic aci...)Show SMILES CCCCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C18H35N3O4/c1-7-8-9-10-11-14(12-21(25)13-22)16(23)19-15(18(2,3)4)17(24)20(5)6/h13-15,25H,7-12H2,1-6H3,(H,19,23)/t14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131289
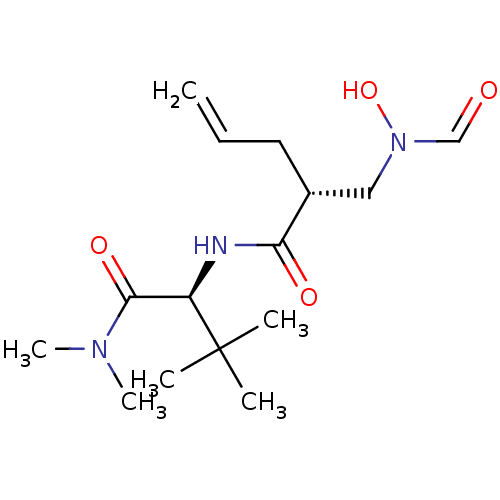
((R)-2-[(Formyl-hydroxy-amino)-methyl]-pent-4-enoic...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@H](CC=C)CN(O)C=O)C(C)(C)C Show InChI InChI=1S/C15H27N3O4/c1-7-8-11(9-18(22)10-19)13(20)16-12(15(2,3)4)14(21)17(5)6/h7,10-12,22H,1,8-9H2,2-6H3,(H,16,20)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131296
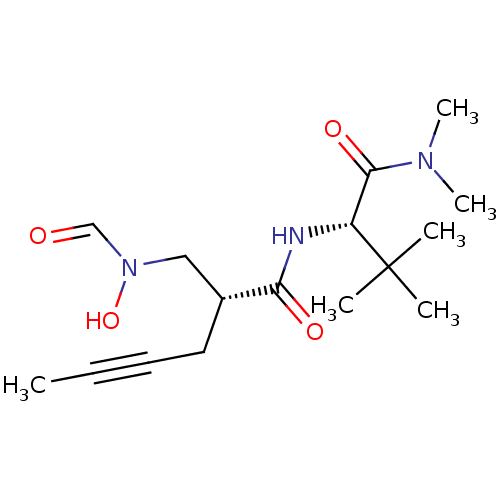
((R)-2-[(Formyl-hydroxy-amino)-methyl]-hex-4-ynoic ...)Show SMILES CC#CC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H27N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,9-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131297
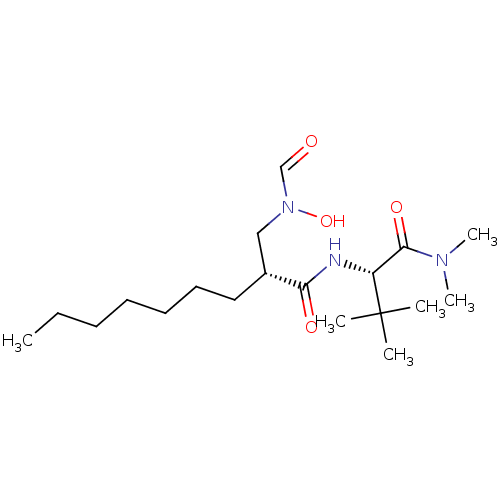
((R)-2-[(Formyl-hydroxy-amino)-methyl]-nonanoic aci...)Show SMILES CCCCCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C19H37N3O4/c1-7-8-9-10-11-12-15(13-22(26)14-23)17(24)20-16(19(2,3)4)18(25)21(5)6/h14-16,26H,7-13H2,1-6H3,(H,20,24)/t15-,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131293
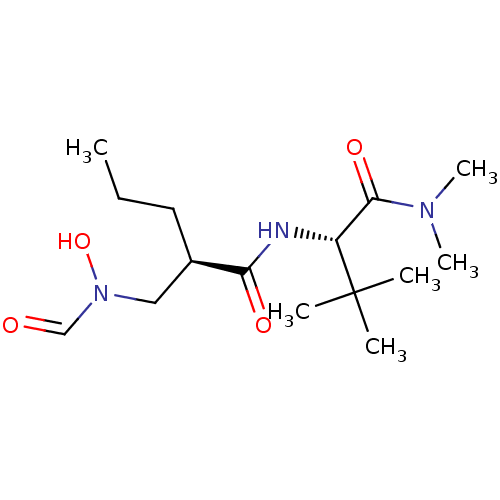
((R)-2-[(Formyl-hydroxy-amino)-methyl]-pentanoic ac...)Show SMILES CCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-7-8-11(9-18(22)10-19)13(20)16-12(15(2,3)4)14(21)17(5)6/h10-12,22H,7-9H2,1-6H3,(H,16,20)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131287
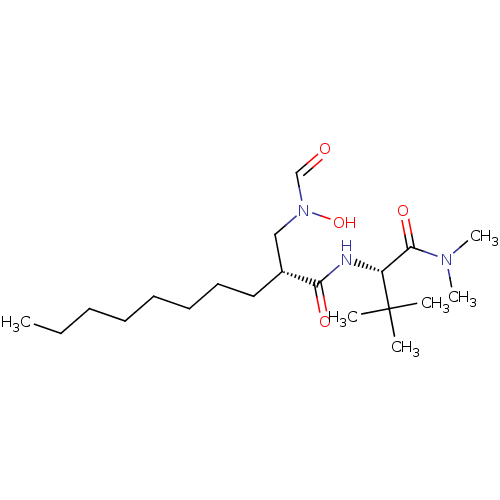
((R)-2-[(Formyl-hydroxy-amino)-methyl]-decanoic aci...)Show SMILES CCCCCCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C20H39N3O4/c1-7-8-9-10-11-12-13-16(14-23(27)15-24)18(25)21-17(20(2,3)4)19(26)22(5)6/h15-17,27H,7-14H2,1-6H3,(H,21,25)/t16-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131306
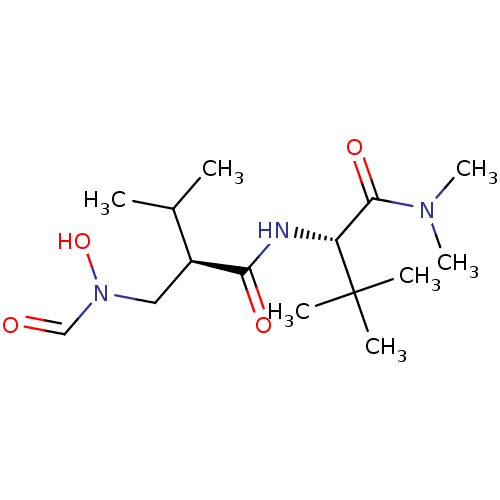
((R)-N-((S)-1-Dimethylcarbamoyl-2,2-dimethyl-propyl...)Show SMILES CC(C)[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-10(2)11(8-18(22)9-19)13(20)16-12(15(3,4)5)14(21)17(6)7/h9-12,22H,8H2,1-7H3,(H,16,20)/t11-,12+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131299

((S)-2-{(R)-2-[(Formyl-hydroxy-amino)-methyl]-butyr...)Show SMILES CC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C14H27N3O4/c1-7-10(8-17(21)9-18)12(19)15-11(14(2,3)4)13(20)16(5)6/h9-11,21H,7-8H2,1-6H3,(H,15,19)/t10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50104500
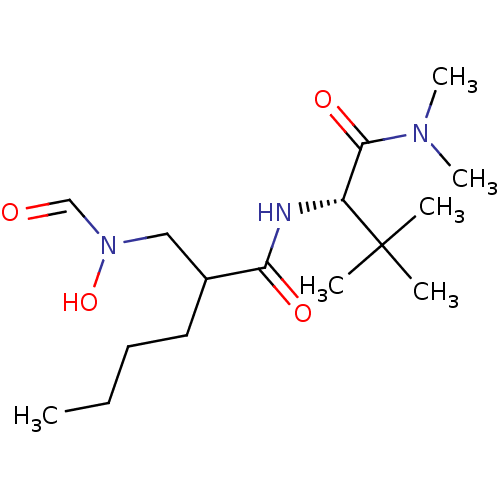
(2-[(Formyl-hydroxy-amino)-methyl]-hexanoic acid ((...)Show SMILES CCCCC(CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12?,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Antibacterial activity of the compound against E. coli Peptide deformylase. Ni |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131308
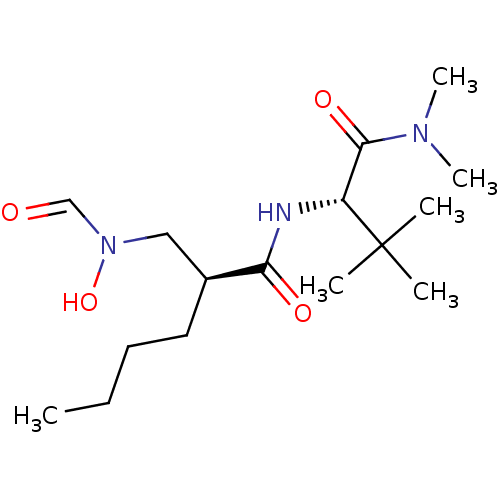
((S)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131305
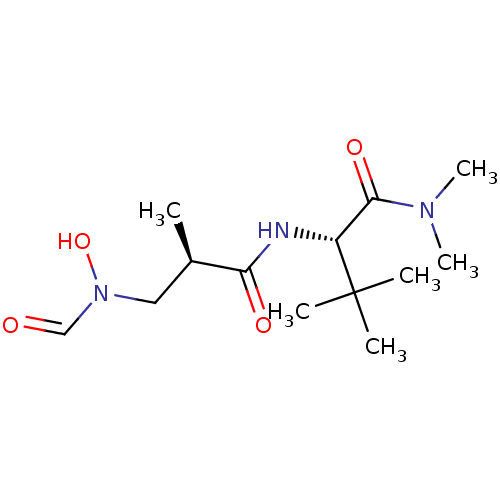
((S)-2-[(R)-3-(Formyl-hydroxy-amino)-2-methyl-propi...)Show SMILES C[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C13H25N3O4/c1-9(7-16(20)8-17)11(18)14-10(13(2,3)4)12(19)15(5)6/h8-10,20H,7H2,1-6H3,(H,14,18)/t9-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121436
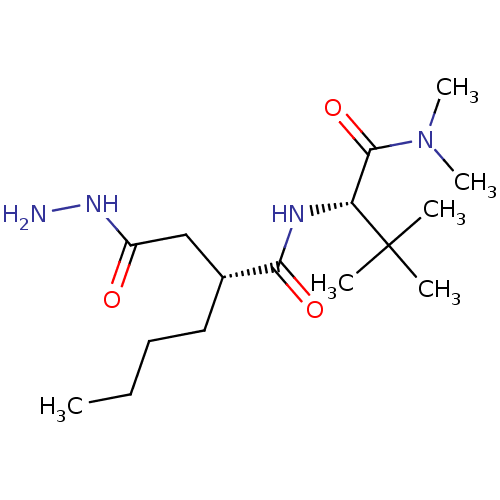
((R)-2-Hydrazinocarbonylmethyl-hexanoic acid ((S)-1...)Show SMILES CCCC[C@H](CC(=O)NN)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H32N4O3/c1-7-8-9-11(10-12(21)19-17)14(22)18-13(16(2,3)4)15(23)20(5)6/h11,13H,7-10,17H2,1-6H3,(H,18,22)(H,19,21)/t11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131286

((S)-2-[(R)-3-(Formyl-hydroxy-amino)-2-(4-methoxy-b...)Show SMILES COc1ccc(C[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C)cc1 Show InChI InChI=1S/C20H31N3O5/c1-20(2,3)17(19(26)22(4)5)21-18(25)15(12-23(27)13-24)11-14-7-9-16(28-6)10-8-14/h7-10,13,15,17,27H,11-12H2,1-6H3,(H,21,25)/t15-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121440
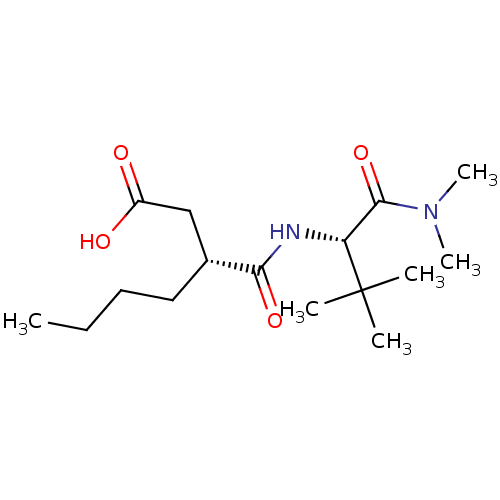
((R)-3-((S)-1-Dimethylcarbamoyl-2,2-dimethyl-propyl...)Show SMILES CCCC[C@H](CC(O)=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H30N2O4/c1-7-8-9-11(10-12(19)20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11,13H,7-10H2,1-6H3,(H,17,21)(H,19,20)/t11-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131309
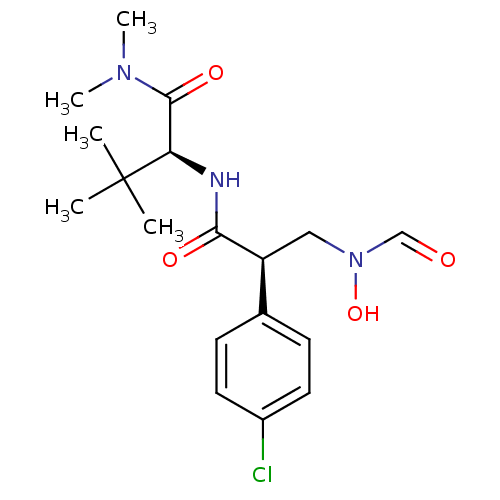
((S)-2-[(R)-2-(4-Chloro-phenyl)-3-(formyl-hydroxy-a...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@@H](CN(O)C=O)c1ccc(Cl)cc1)C(C)(C)C Show InChI InChI=1S/C18H26ClN3O4/c1-18(2,3)15(17(25)21(4)5)20-16(24)14(10-22(26)11-23)12-6-8-13(19)9-7-12/h6-9,11,14-15,26H,10H2,1-5H3,(H,20,24)/t14-,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131298

((R)-2-[1-(Formyl-hydroxy-amino)-ethyl]-hexanoic ac...)Show SMILES CCCC[C@H](C(C)N(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C17H33N3O4/c1-8-9-10-13(12(2)20(24)11-21)15(22)18-14(17(3,4)5)16(23)19(6)7/h11-14,24H,8-10H2,1-7H3,(H,18,22)/t12?,13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121443

(CHEMBL120357 | [2-((S)-1-Dimethylcarbamoyl-2,2-dim...)Show SMILES CCCCC(CP(O)(O)=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C15H31N2O5P/c1-7-8-9-11(10-23(20,21)22)13(18)16-12(15(2,3)4)14(19)17(5)6/h11-12H,7-10H2,1-6H3,(H,16,18)(H2,20,21,22)/t11?,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121437
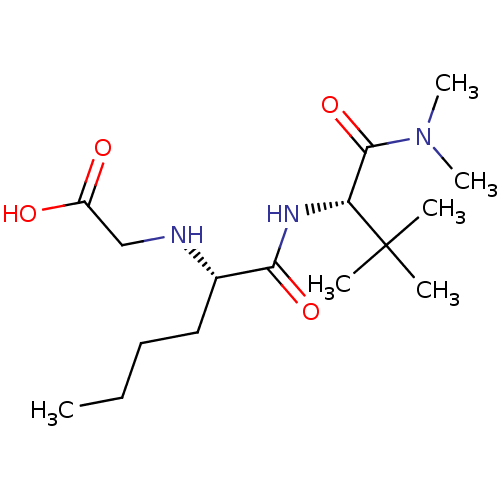
(CHEMBL422076 | [(S)-1-((S)-1-Dimethylcarbamoyl-2,2...)Show SMILES CCCC[C@H](NCC(O)=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-11(17-10-12(20)21)14(22)18-13(16(2,3)4)15(23)19(5)6/h11,13,17H,7-10H2,1-6H3,(H,18,22)(H,20,21)/t11-,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121438
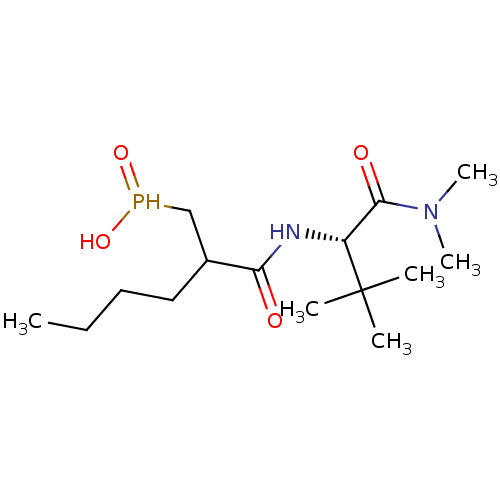
(CHEMBL120291 | [2-((S)-1-Dimethylcarbamoyl-2,2-dim...)Show SMILES CCCCC(CP(O)=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C15H31N2O4P/c1-7-8-9-11(10-22(20)21)13(18)16-12(15(2,3)4)14(19)17(5)6/h11-12,22H,7-10H2,1-6H3,(H,16,18)(H,20,21)/t11?,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131294

((S)-2-{(R)-2-[(Formyl-hydroxy-amino)-methyl]-3-pip...)Show SMILES CN(C)C(=O)[C@@H](NC(=O)[C@@H](CN(O)C=O)CN1CCCCC1)C(C)(C)C Show InChI InChI=1S/C18H34N4O4/c1-18(2,3)15(17(25)20(4)5)19-16(24)14(12-22(26)13-23)11-21-9-7-6-8-10-21/h13-15,26H,6-12H2,1-5H3,(H,19,24)/t14-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121441

((R)-2-(N-Hydroxycarbamimidoylmethyl)-hexanoic acid...)Show SMILES CCCCC(CC(=N)NO)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H32N4O3/c1-7-8-9-11(10-12(17)19-23)14(21)18-13(16(2,3)4)15(22)20(5)6/h11,13,23H,7-10H2,1-6H3,(H2,17,19)(H,18,21)/t11?,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50131300

((R)-2-[2-(Formyl-hydroxy-amino)-ethyl]-hexanoic ac...)Show SMILES CCCC[C@H](CCN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C17H33N3O4/c1-7-8-9-13(10-11-20(24)12-21)15(22)18-14(17(2,3)4)16(23)19(5)6/h12-14,24H,7-11H2,1-6H3,(H,18,22)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition against Escherichia coli peptide deformylase |
Bioorg Med Chem Lett 13: 2709-13 (2003)
BindingDB Entry DOI: 10.7270/Q2XW4J6S |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121446

((R)-2-Formylaminomethyl-hexanoic acid ((S)-1-dimet...)Show SMILES CCCC[C@H](CNC=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O3/c1-7-8-9-12(10-17-11-20)14(21)18-13(16(2,3)4)15(22)19(5)6/h11-13H,7-10H2,1-6H3,(H,17,20)(H,18,21)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Peptide deformylase, mitochondrial
(Homo sapiens (Human)) | BDBM50121439
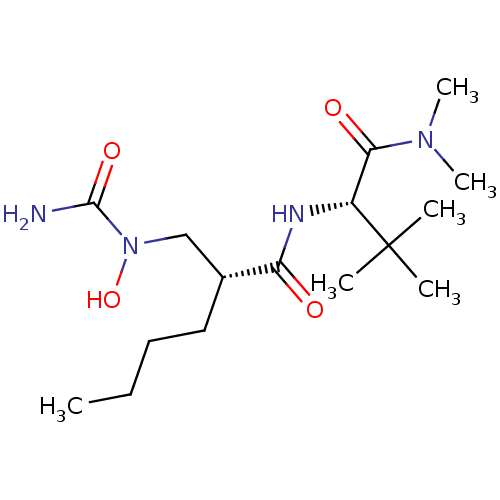
((2R)-2-{[(aminocarbonyl)(hydroxy)amino]methyl}-N-{...)Show SMILES CCCC[C@H](CN(O)C(N)=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H32N4O4/c1-7-8-9-11(10-20(24)15(17)23)13(21)18-12(16(2,3)4)14(22)19(5)6/h11-12,24H,7-10H2,1-6H3,(H2,17,23)(H,18,21)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of peptide deformylase, PDF.Ni of Escherichia coli |
Bioorg Med Chem Lett 12: 3595-9 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G4H |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-3 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50089194

((R)-N*4*-Hydroxy-N*1*-[(S)-1-((S)-2-hydroxymethyl-...)Show SMILES CCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1CO Show InChI InChI=1S/C19H35N3O5/c1-4-5-6-8-14(11-16(24)21-27)18(25)20-17(13(2)3)19(26)22-10-7-9-15(22)12-23/h13-15,17,23,27H,4-12H2,1-3H3,(H,20,25)(H,21,24)/t14-,15+,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for the inhibition of Neutral endopeptidase |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for the inhibition of Neutral endopeptidase |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50104500

(2-[(Formyl-hydroxy-amino)-methyl]-hexanoic acid ((...)Show SMILES CCCCC(CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12?,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-7 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50104501

((R)-2-[(Formyl-hydroxy-amino)-methyl]-hexanoic aci...)Show SMILES CCCC[C@H](CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against Angiotensin I converting enzyme |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104500

(2-[(Formyl-hydroxy-amino)-methyl]-hexanoic acid ((...)Show SMILES CCCCC(CN(O)C=O)C(=O)N[C@H](C(=O)N(C)C)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-7-8-9-12(10-19(23)11-20)14(21)17-13(16(2,3)4)15(22)18(5)6/h11-13,23H,7-10H2,1-6H3,(H,17,21)/t12?,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 2585-8 (2001)
BindingDB Entry DOI: 10.7270/Q2SN0886 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data