

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50470923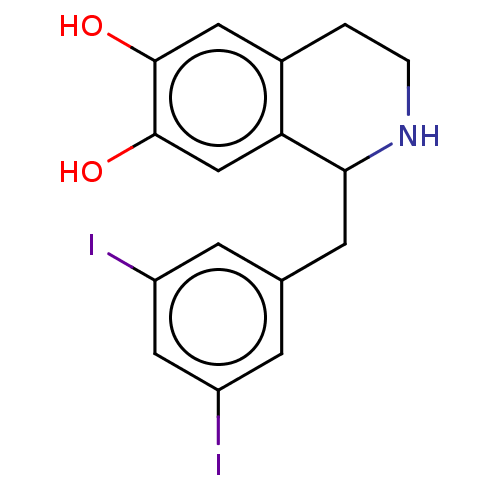 (CHEMBL122757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis Curated by ChEMBL | Assay Description Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... | J Med Chem 39: 3701-11 (1996) Article DOI: 10.1021/jm960208o BindingDB Entry DOI: 10.7270/Q2Q52SBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140558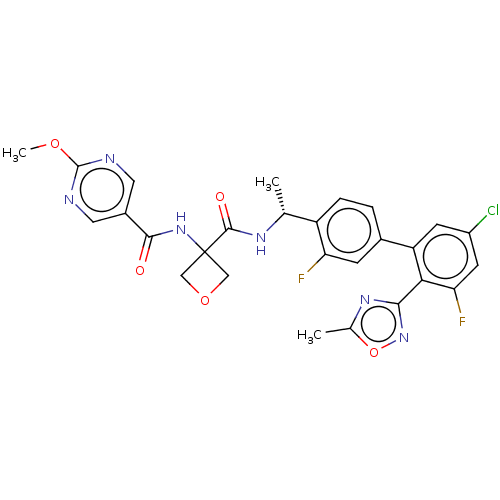 (US8912221, 31) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140539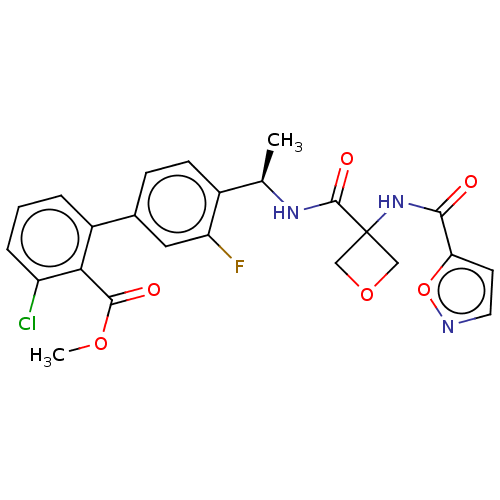 (US8912221, 12) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140552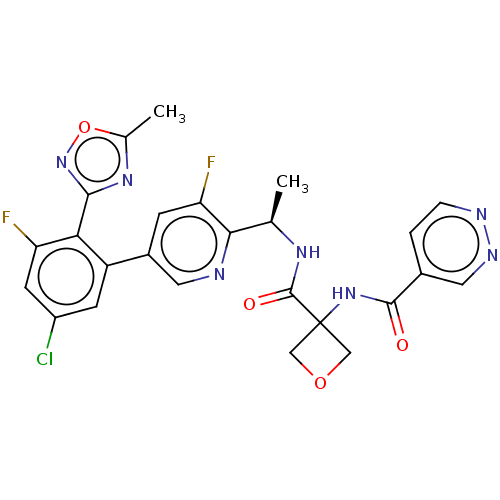 (US8912221, 25) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140540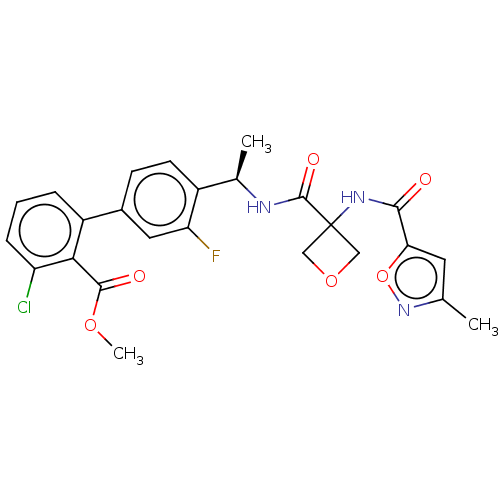 (US8912221, 13) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140550 (US8912221, 23) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140542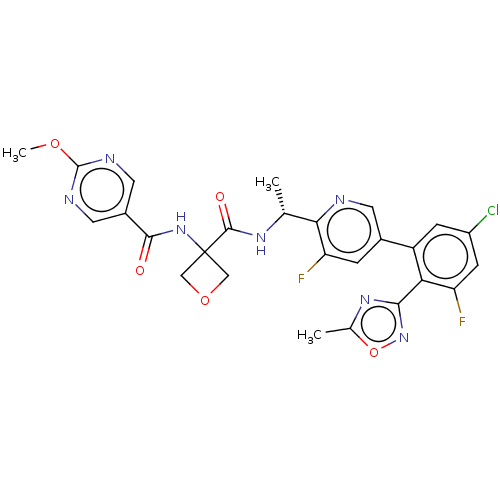 (US8912221, 15) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140567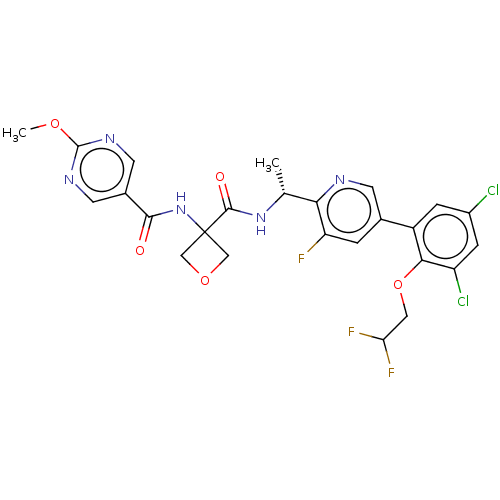 (US8912221, 40) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140532 (US8912221, 5) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140571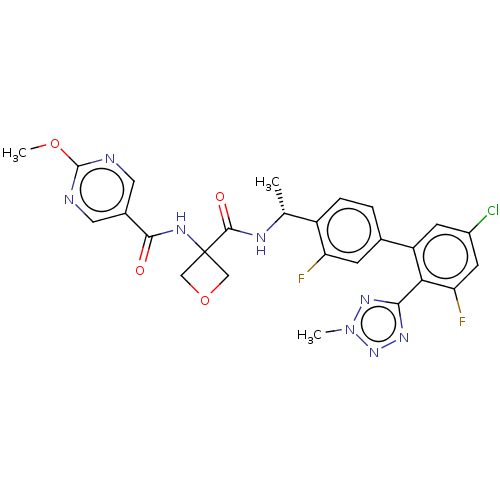 (US8912221, 44) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140563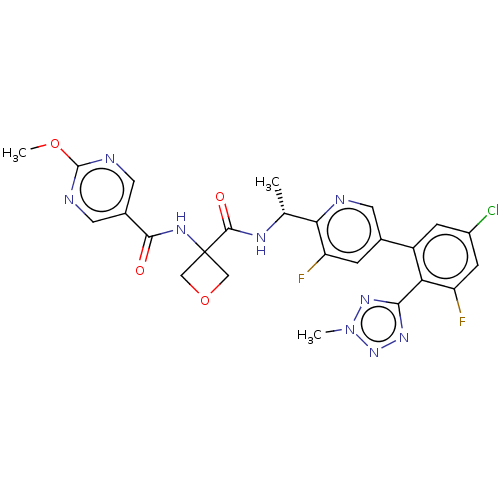 (US8912221, 36) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140551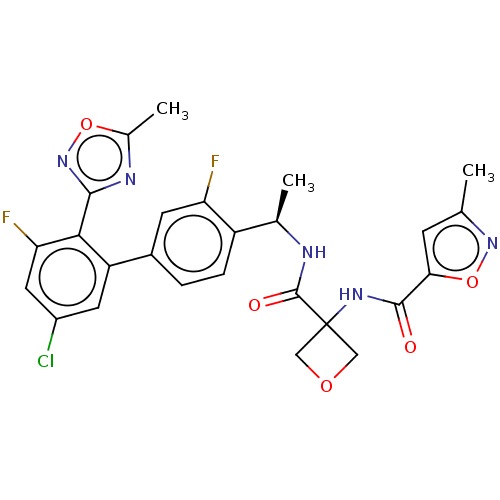 (US8912221, 24) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140557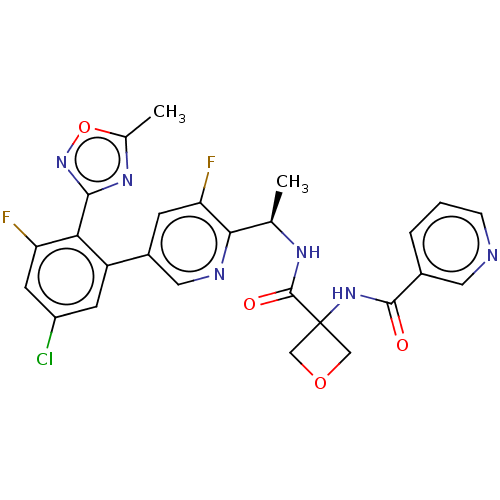 (US8912221, 30) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140570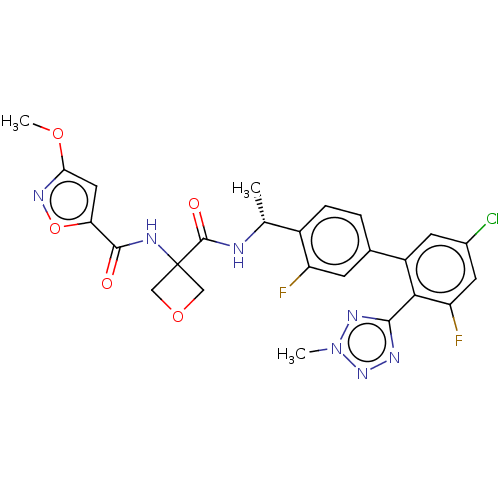 (US8912221, 43) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140541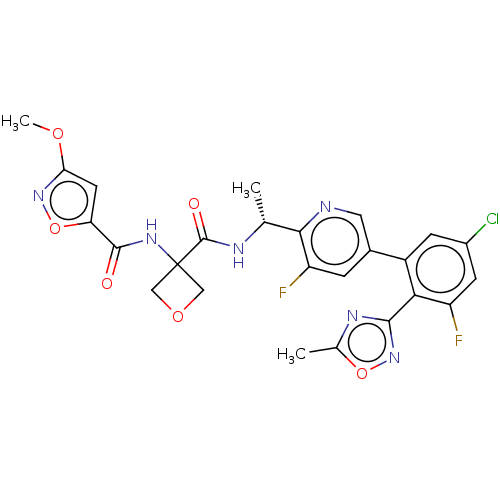 (US8912221, 14) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140555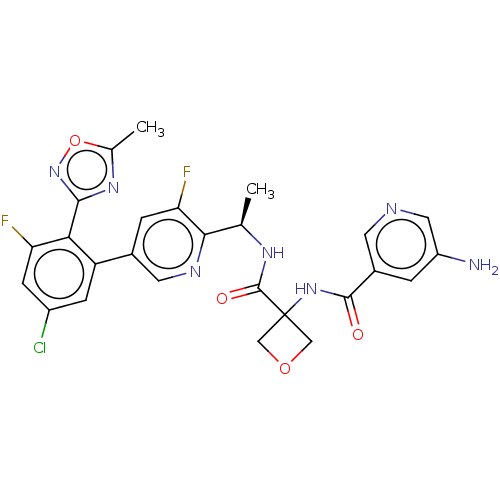 (US8912221, 28) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50470918 (CHEMBL123596) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis Curated by ChEMBL | Assay Description Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... | J Med Chem 39: 3701-11 (1996) Article DOI: 10.1021/jm960208o BindingDB Entry DOI: 10.7270/Q2Q52SBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50470917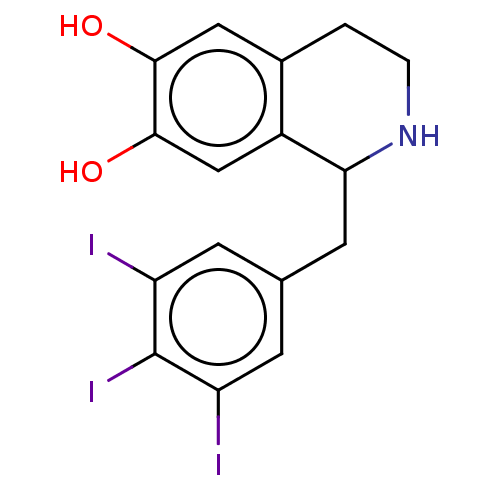 (CHEMBL120278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis Curated by ChEMBL | Assay Description Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... | J Med Chem 39: 3701-11 (1996) Article DOI: 10.1021/jm960208o BindingDB Entry DOI: 10.7270/Q2Q52SBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140547 (US8912221, 20) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140543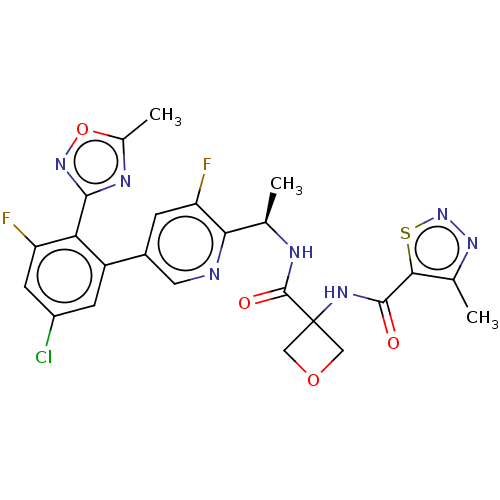 (US8912221, 16) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140534 (US8912221, 7) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140549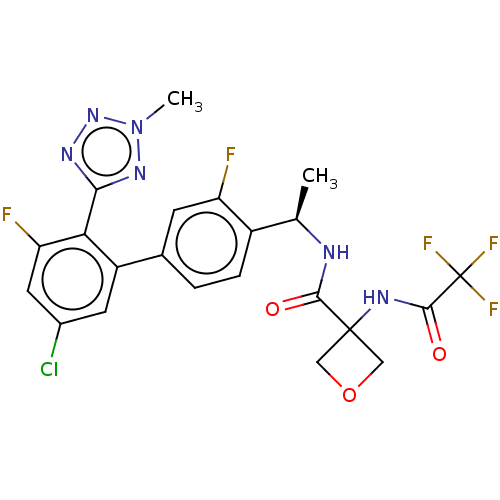 (US8912221, 22) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208974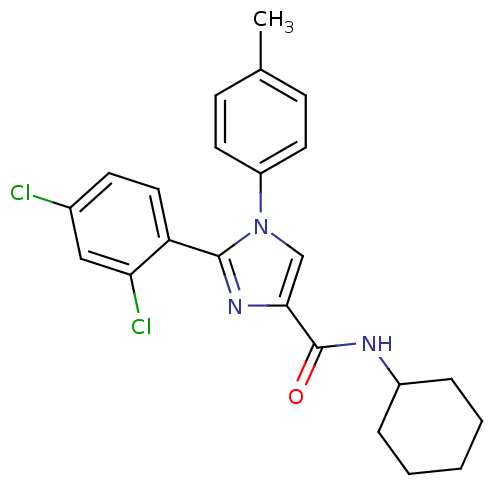 (CHEMBL390543 | N-cyclohexyl-2-(2,4-dichlorophenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM82487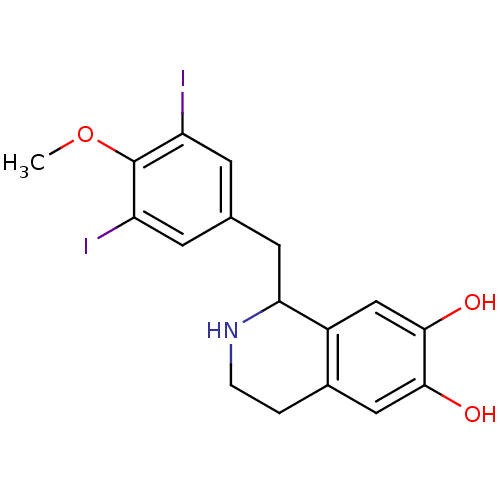 (TMQ, 3',5'-Diiodo) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis Curated by ChEMBL | Assay Description Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... | J Med Chem 39: 3701-11 (1996) Article DOI: 10.1021/jm960208o BindingDB Entry DOI: 10.7270/Q2Q52SBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM82487 (TMQ, 3',5'-Diiodo) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Curated by ChEMBL | Assay Description Binding Affinity against human beta-2 adrenergic receptor expressed in Chinese hamster ovary(CHO) cells was measured by using [125I]ICYP radioligand | J Med Chem 42: 2287-94 (1999) Article DOI: 10.1021/jm990012z BindingDB Entry DOI: 10.7270/Q2057JN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50470916 (CHEMBL121704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee-Memphis Curated by ChEMBL | Assay Description Binding affinity for human Beta-2 adrenergic receptor expressed in CHO cells by radioligand competition binding assays using [125I]iodocyanopindolol ... | J Med Chem 39: 3701-11 (1996) Article DOI: 10.1021/jm960208o BindingDB Entry DOI: 10.7270/Q2Q52SBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198507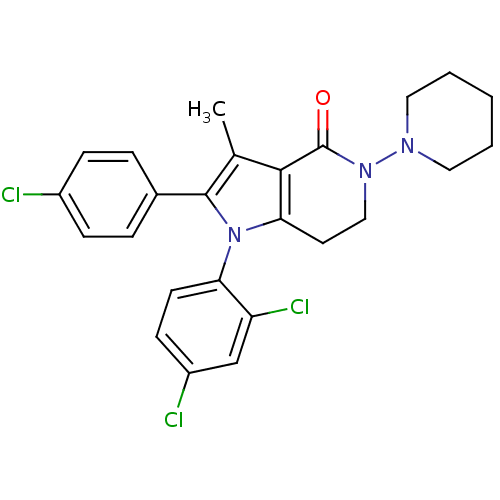 (2-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140561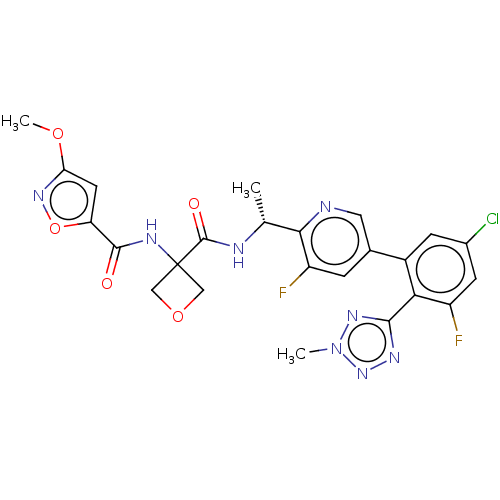 (US8912221, 34) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208948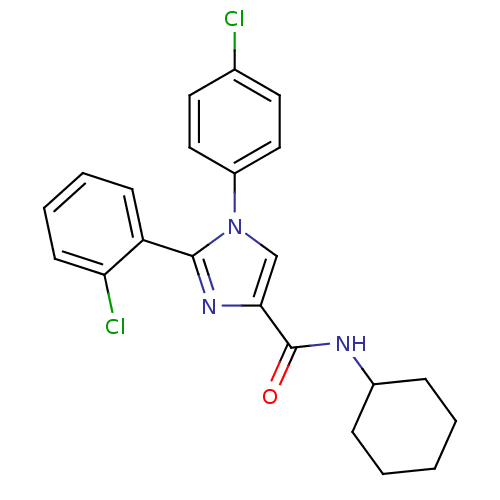 (2-(2-chlorophenyl)-1-(4-chlorophenyl)-N-cyclohexyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140559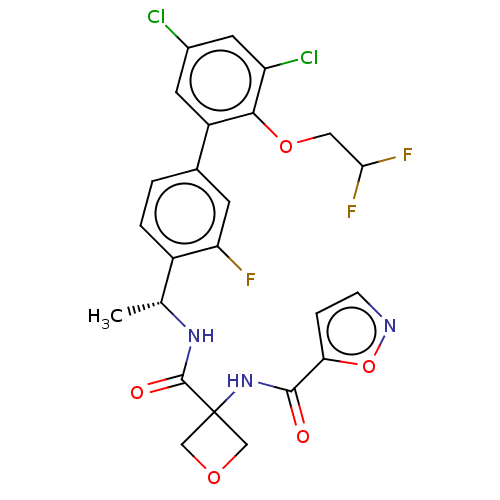 (US8912221, 32) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140530 (US8912221, 3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140531 (US8912221, 4) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140538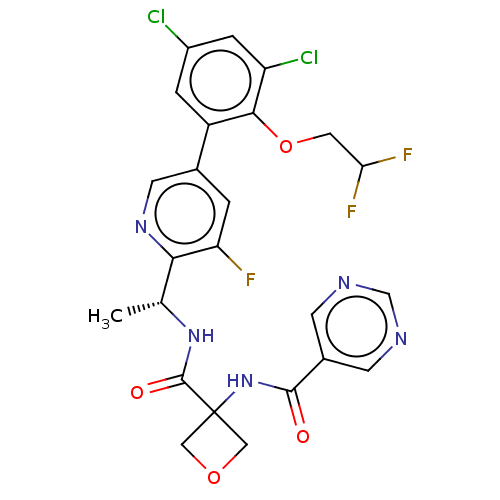 (US8912221, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107177 (US8592426, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140528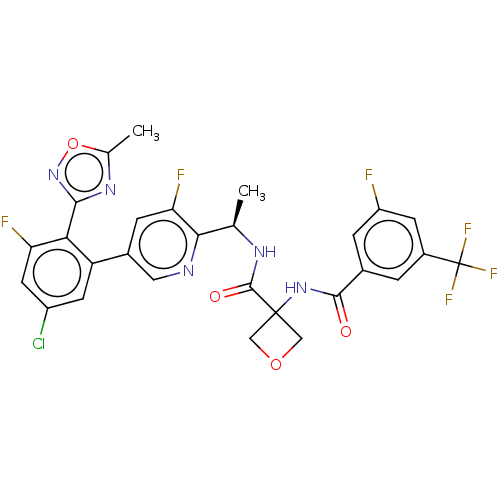 (US8912221, 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198532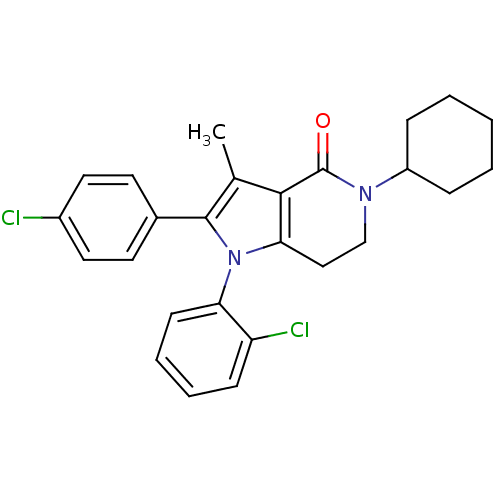 (1-(2-chlorophenyl)-2-(4-chlorophenyl)-5-cyclohexyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107189 (US8592426, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140545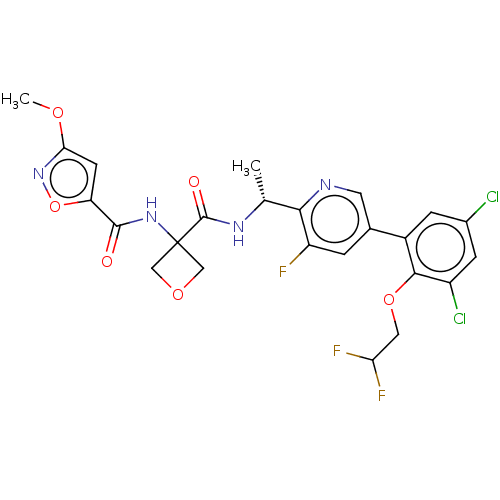 (US8912221, 18) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107176 (US8592426, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140562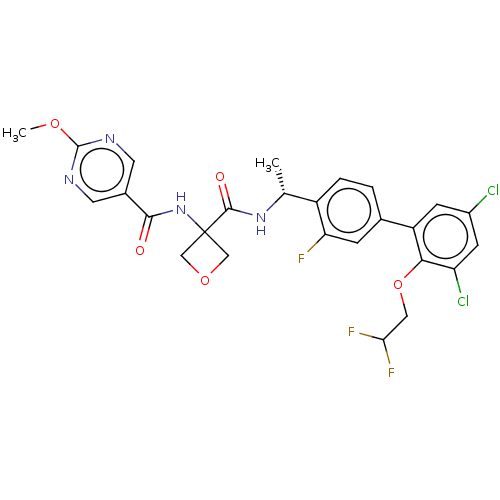 (US8912221, 35) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107209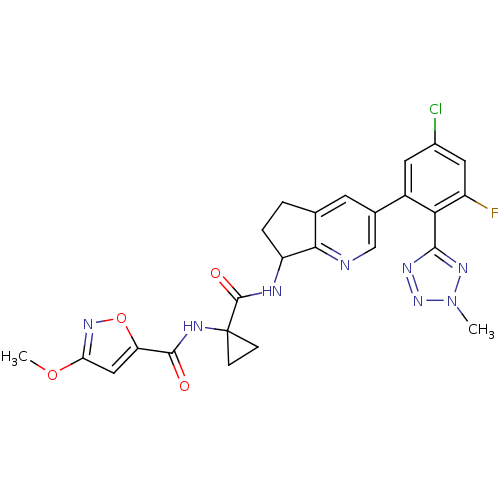 (US8592426, 59) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198519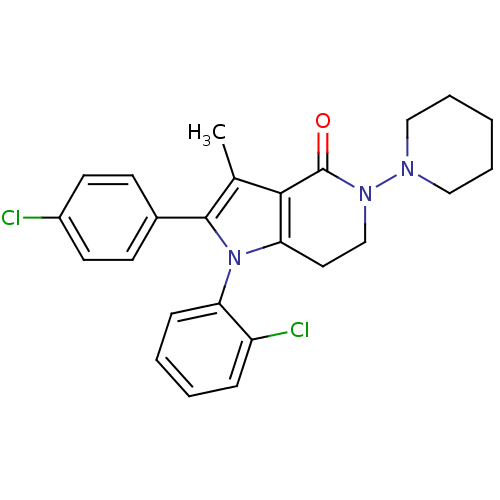 (1-(2-chlorophenyl)-2-(4-chlorophenyl)-3-methyl-5-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM140564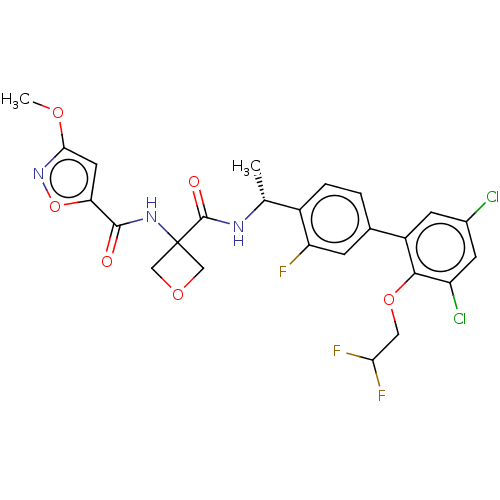 (US8912221, 37) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kall... | US Patent US8912221 (2014) BindingDB Entry DOI: 10.7270/Q2668BWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107178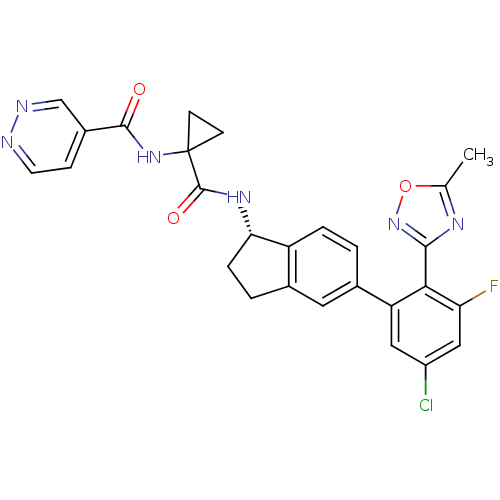 (US8592426, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107203 (US8592426, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208975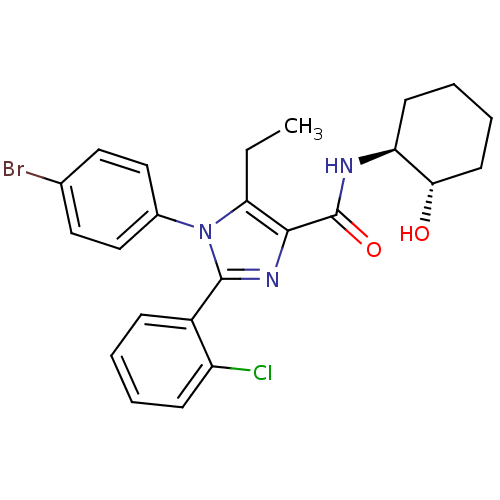 (1-(4-bromophenyl)-2-(2-chlorophenyl)-5-ethyl-N-((1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140237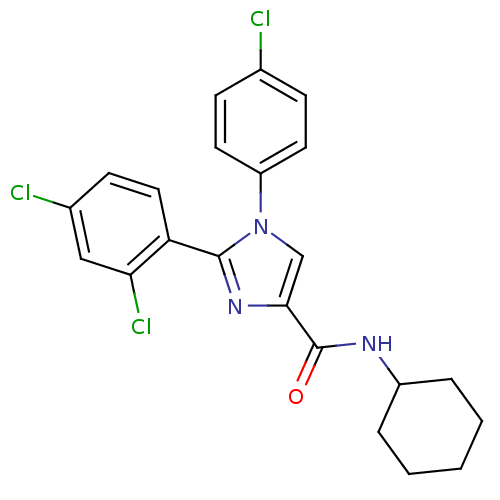 (1-(4-Chloro-phenyl)-2-(2,4-dichloro-phenyl)-1H-imi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107181 (US8592426, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kininogen-1 (Homo sapiens (Human)) | BDBM107190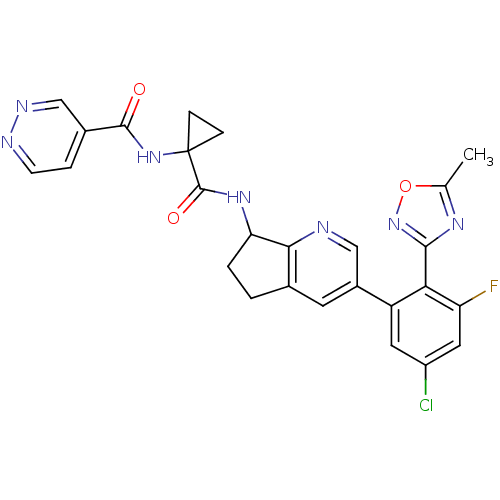 (US8592426, 116 | US8592426, 117 | US8592426, 39/40...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann—La Roche Inc. US Patent | Assay Description Binding assay using Bradykinin-1 receptor. | US Patent US8592426 (2013) BindingDB Entry DOI: 10.7270/Q2WH2NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 353 total ) | Next | Last >> |