

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263324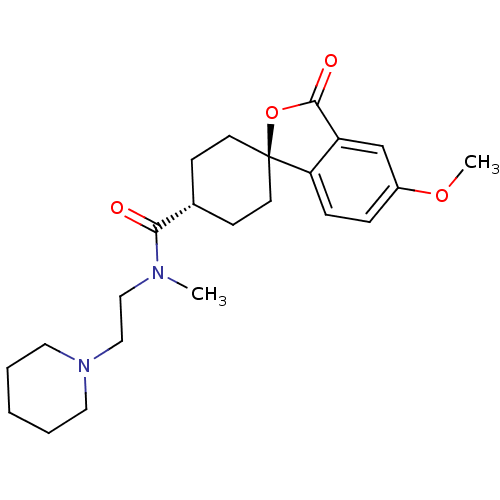 (CHEMBL513893 | trans-5'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263323 (CHEMBL514217 | trans-3'-Oxo-3'H-spiro[cyclohexane-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263357 (CHEMBL478434 | trans-7'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263325 (CHEMBL507921 | trans-6'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263321 (CHEMBL476962 | N-methyl-3-oxo-N-[2-(piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264067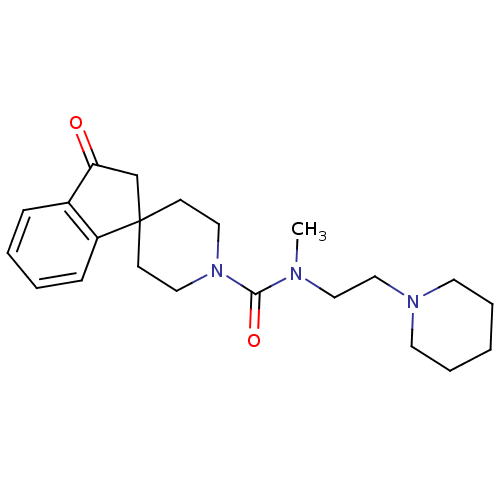 (CHEMBL521798 | N-methyl-3-oxo-N-[2-(piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264069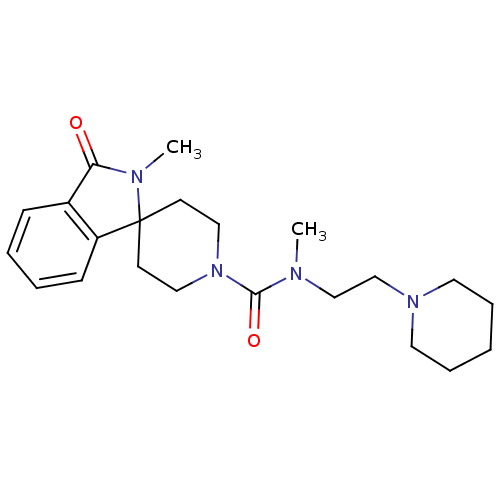 (CHEMBL491806 | N,2-dimethyl-3-oxo-N-[2-(piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264068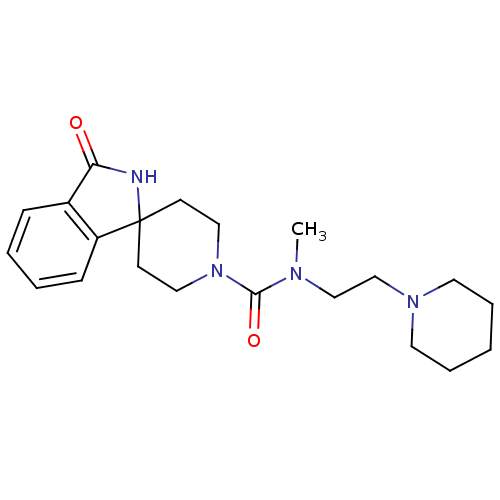 (CHEMBL504191 | N-methyl-3-oxo-N-[2-(piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264066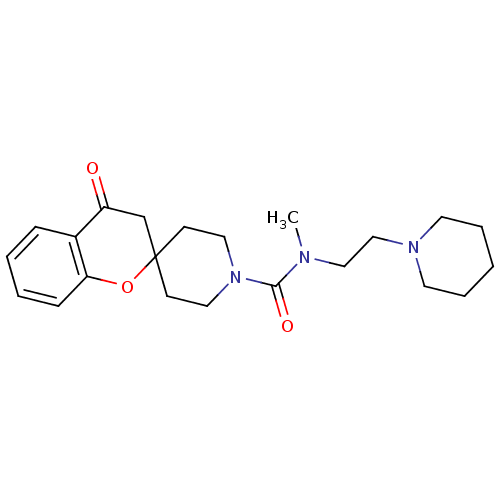 (CHEMBL491805 | N-methyl-4-oxo-N-[2-(piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263900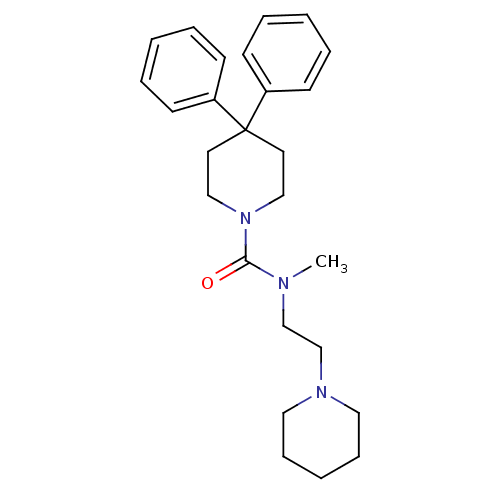 (CHEMBL523992 | N-methyl-4,4-diphenyl-N-(2-(piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263844 (CHEMBL491614 | N-methyl-4-(naphthalen-2-yl)-N-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264012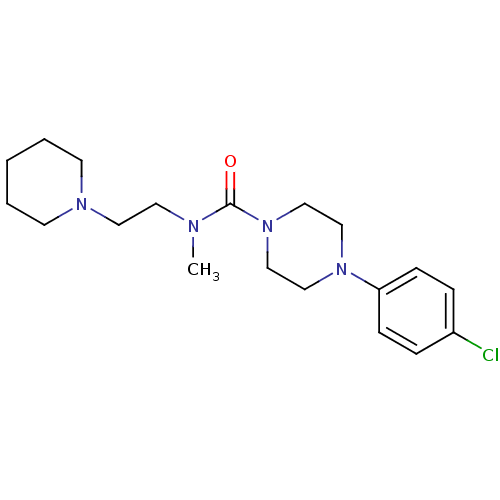 (4-(4-chlorophenyl)-N-methyl-N-(2-(piperidin-1-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263898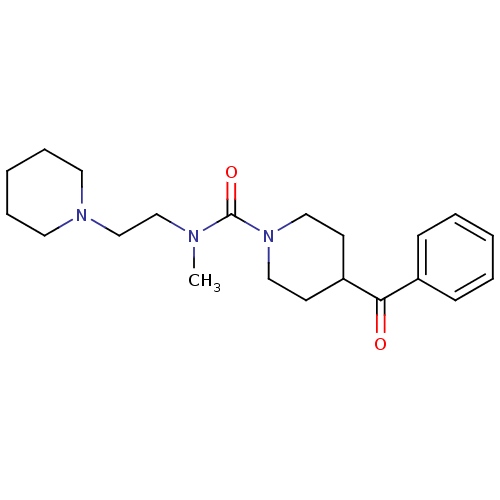 (4-benzoyl-N-methyl-N-(2-(piperidin-1-yl)ethyl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263948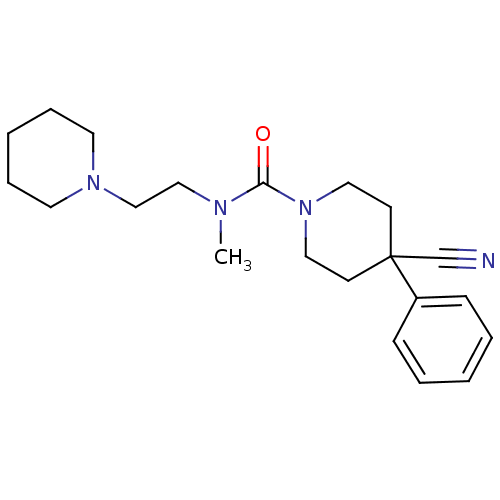 (4-cyano-N-methyl-4-phenyl-N-(2-(piperidin-1-yl)eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263952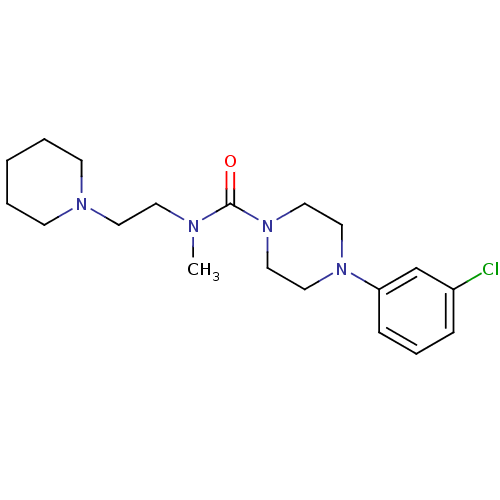 (4-(3-chlorophenyl)-N-methyl-N-(2-(piperidin-1-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263845 (CHEMBL490195 | N-methyl-4-(naphthalen-1-yl)-N-(2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264065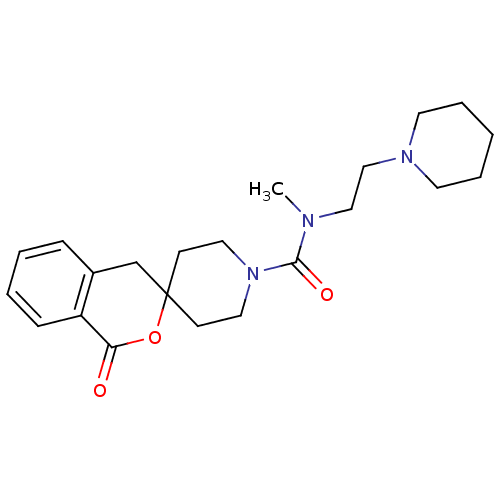 (CHEMBL489973 | N-methyl-1-oxo-N-[2-(piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263322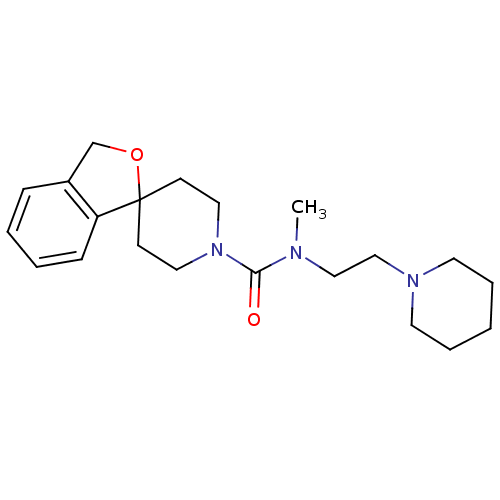 (CHEMBL476963 | N-methyl-N-[2-(piperidin-1-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263897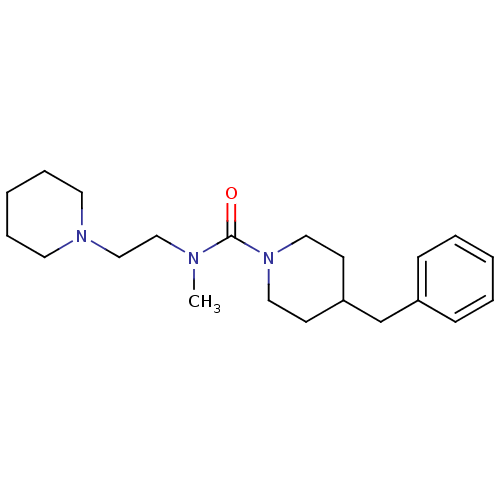 (4-benzyl-N-methyl-N-(2-(piperidin-1-yl)ethyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263950 (CHEMBL490612 | N-methyl-4-phenyl-N-(2-(piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263899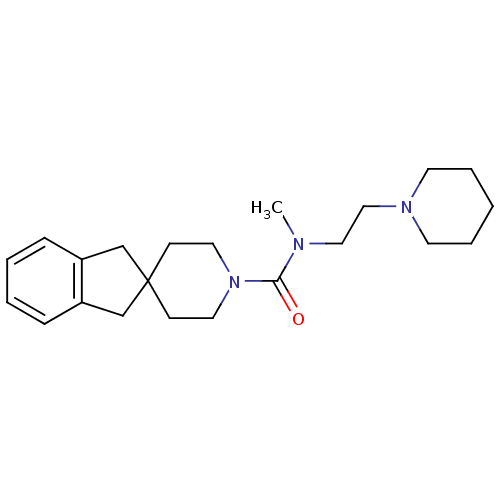 (CHEMBL492030 | N-methyl-N-[2-(piperidin-1-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264016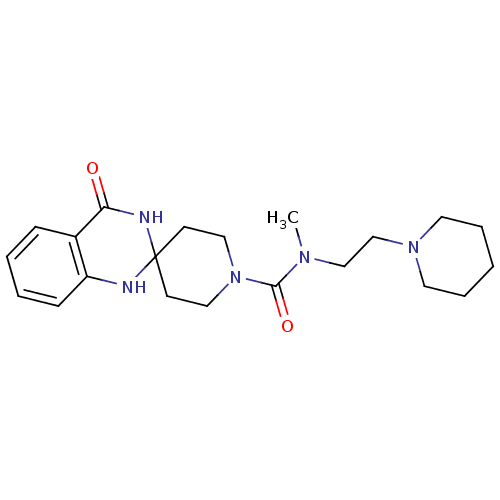 (CHEMBL490993 | N-methyl-4'-oxo-N-[2-(piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263896 (CHEMBL501876 | N-methyl-N-(2-(piperidin-1-yl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264014 (3-Oxo-3,4-dihydro-2H-spiro[isoquinoline-1,4'-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 783 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263846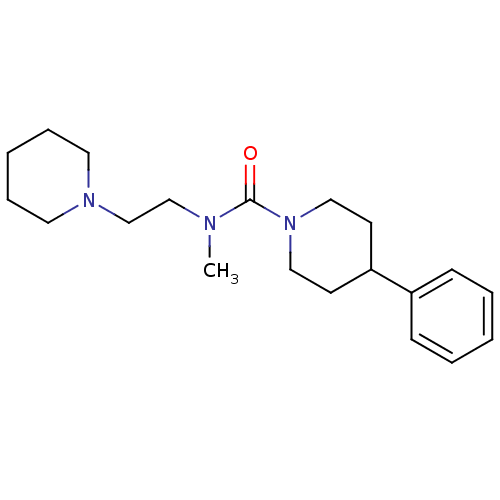 (CHEMBL490400 | N-methyl-4-phenyl-N-(2-(piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 893 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263951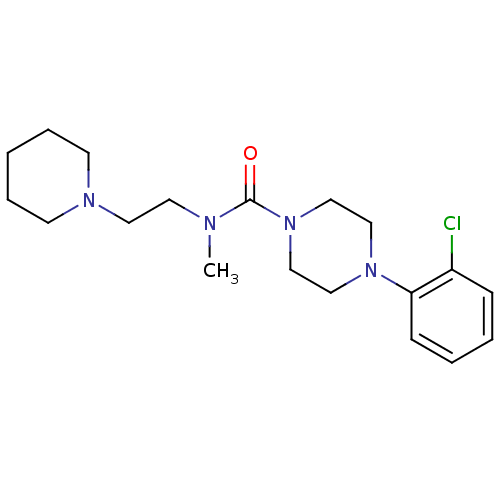 (4-(2-chlorophenyl)-N-methyl-N-(2-(piperidin-1-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264015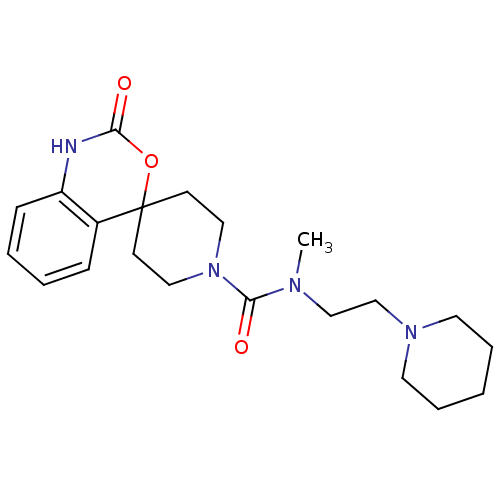 (CHEMBL523635 | N-methyl-2-oxo-N-[2-(piperidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263949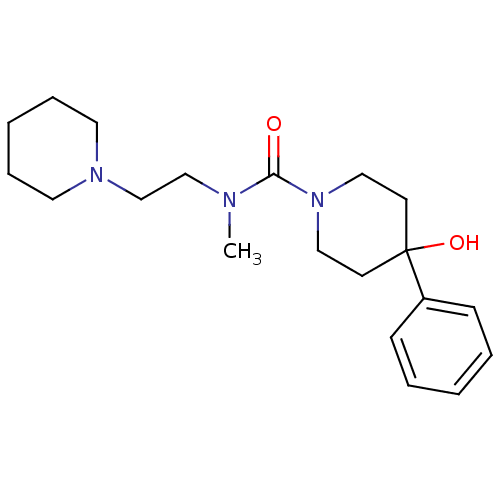 (4-hydroxy-N-methyl-4-phenyl-N-(2-(piperidin-1-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50264013 (4-tert-butyl-N-methyl-N-(2-(piperidin-1-yl)ethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50263323 (CHEMBL514217 | trans-3'-Oxo-3'H-spiro[cyclohexane-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of histamine H4 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50263324 (CHEMBL513893 | trans-5'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of histamine H1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50263324 (CHEMBL513893 | trans-5'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50263323 (CHEMBL514217 | trans-3'-Oxo-3'H-spiro[cyclohexane-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50263323 (CHEMBL514217 | trans-3'-Oxo-3'H-spiro[cyclohexane-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of histamine H1 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50263323 (CHEMBL514217 | trans-3'-Oxo-3'H-spiro[cyclohexane-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50263324 (CHEMBL513893 | trans-5'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of histamine H4 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50263324 (CHEMBL513893 | trans-5'-Methoxy-3'-oxo-3'H-spiro[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | Bioorg Med Chem Lett 18: 5101-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.125 BindingDB Entry DOI: 10.7270/Q2PC3268 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248270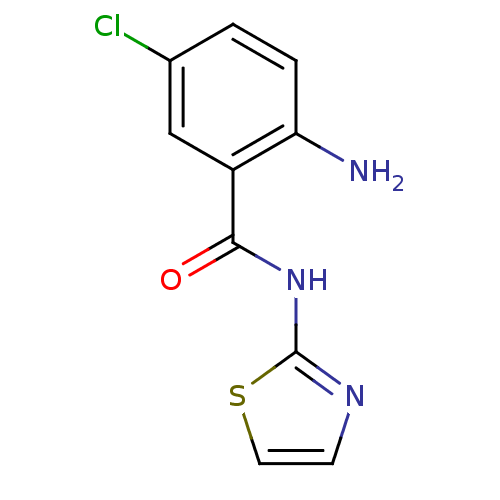 (2-amino-5-chloro-N-(thiazol-2-yl)benzamide | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248270 (2-amino-5-chloro-N-(thiazol-2-yl)benzamide | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248271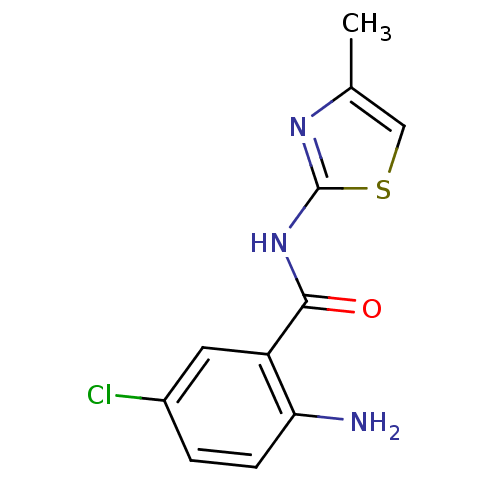 (2-amino-5-chloro-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248271 (2-amino-5-chloro-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248272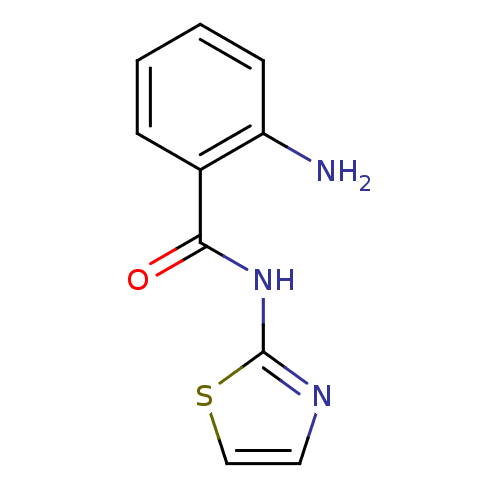 (2-amino-N-(thiazol-2-yl)benzamide | CHEMBL474749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248272 (2-amino-N-(thiazol-2-yl)benzamide | CHEMBL474749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248273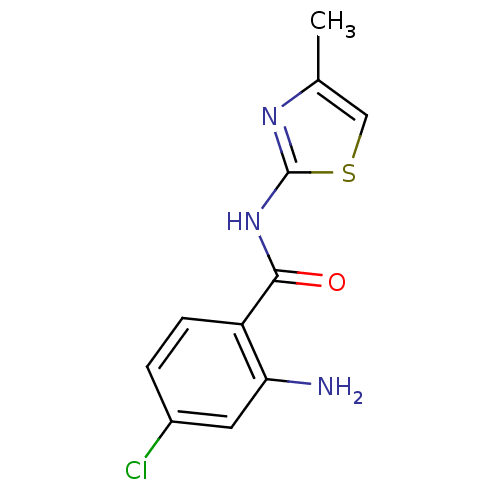 (2-amino-4-chloro-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248273 (2-amino-4-chloro-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50186320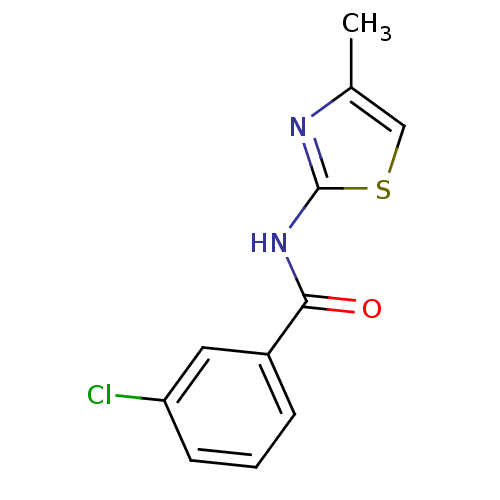 (3-chloro-N-(4-methylthiazol-2-yl)benzamide | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50186320 (3-chloro-N-(4-methylthiazol-2-yl)benzamide | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248274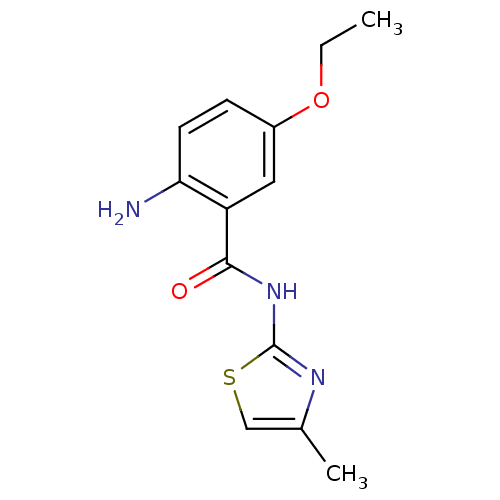 (2-amino-5-ethoxy-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248274 (2-amino-5-ethoxy-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hexokinase-4 (Homo sapiens (Human)) | BDBM50248325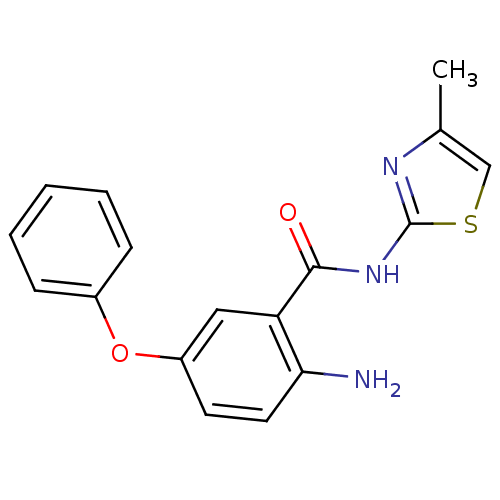 (2-amino-N-(4-methylthiazol-2-yl)-5-phenoxybenzamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucose | Bioorg Med Chem Lett 19: 1357-60 (2009) Article DOI: 10.1016/j.bmcl.2009.01.053 BindingDB Entry DOI: 10.7270/Q2PC3285 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 177 total ) | Next | Last >> |