

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 25 | -44.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 190 | -39.0 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 230 | -38.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86023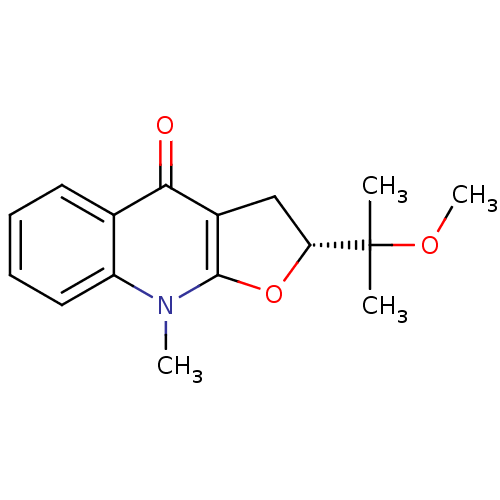 (Methyl isoplatydesmine, 4) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM86023 (Methyl isoplatydesmine, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM86022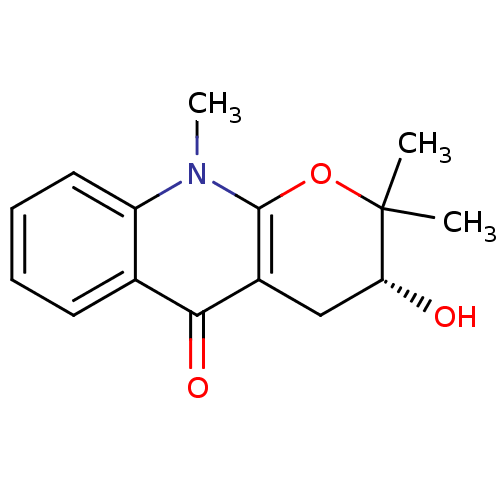 (Ribalinine, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.20E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86022 (Ribalinine, 3) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.00E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM86021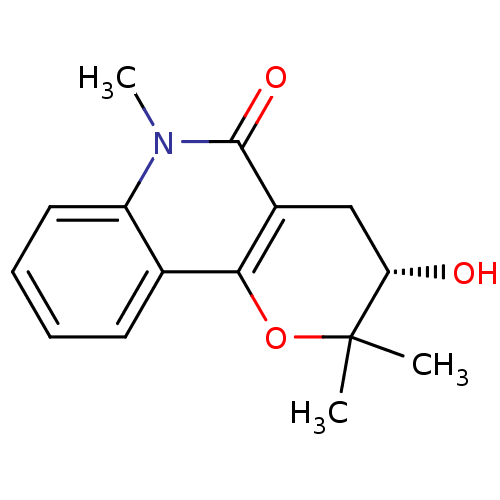 (2,2,6-Trimethyl-3,4,5,6-tetrahydro-2H-pyrano[3,2-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.00E+4 | -23.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM86021 (2,2,6-Trimethyl-3,4,5,6-tetrahydro-2H-pyrano[3,2-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | -23.0 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
University of Karachi | Assay Description AChE and BChE inhibitory activities were measured in vitro by a modified spectrophotometric method. all the inhibition studies were performed using ... | J Enzyme Inhib Med Chem 21: 703-10 (2006) Article DOI: 10.1080/14756360600889708 BindingDB Entry DOI: 10.7270/Q26D5RJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman method | J Nat Prod 66: 739-42 (2003) Article DOI: 10.1021/np020446o BindingDB Entry DOI: 10.7270/Q2862G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum enoyl-ACP reductase assessed as oxidation of NADH to NAD+ after 10 mins by spectrophotometric analysis | Bioorg Med Chem Lett 22: 610-2 (2011) Article DOI: 10.1016/j.bmcl.2011.10.072 BindingDB Entry DOI: 10.7270/Q2K35V3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 857 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of horse BChE | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 857 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) | J Nat Prod 66: 739-42 (2003) Article DOI: 10.1021/np020446o BindingDB Entry DOI: 10.7270/Q2862G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM91713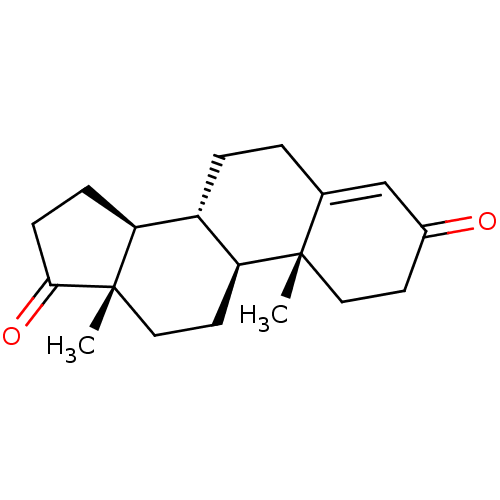 (Androst-4-en-3,17-dione, 2 | Androst-4-ene-3,17-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135147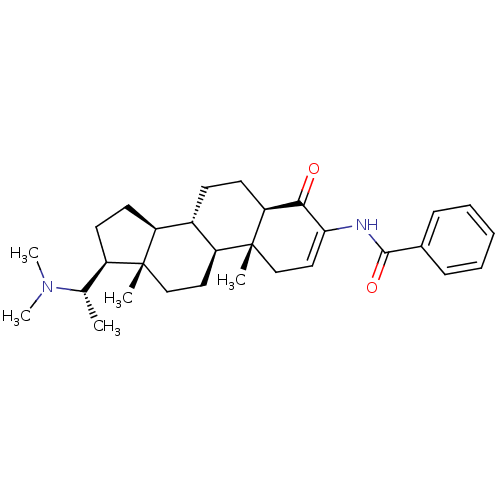 ((+)-axillaridine A | 14-(1-dimethylaminoethyl)-2,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242345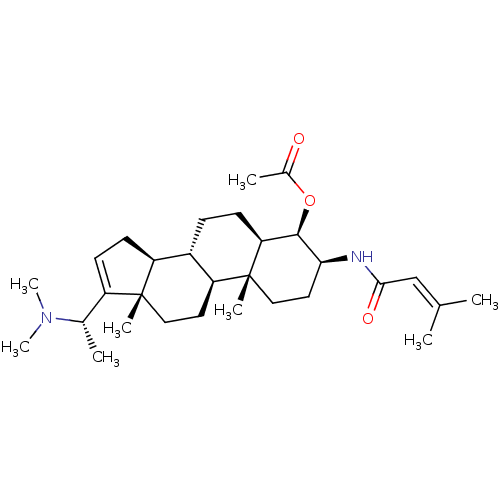 (CHEMBL460447 | [(20S)-20-(N,N-dimethylamino)-3 bet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135146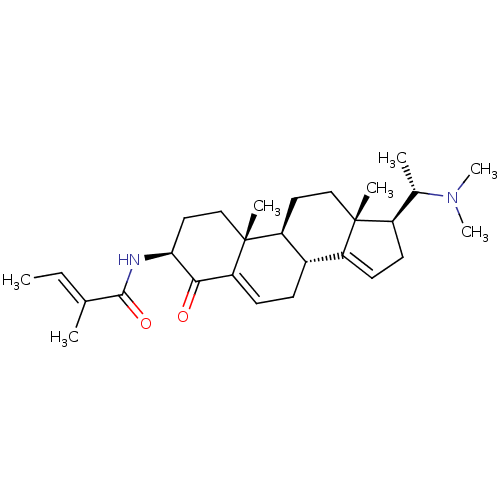 ((E)-2-Methyl-but-2-enoic acid [(3S,8R,9S,10R,13R,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135148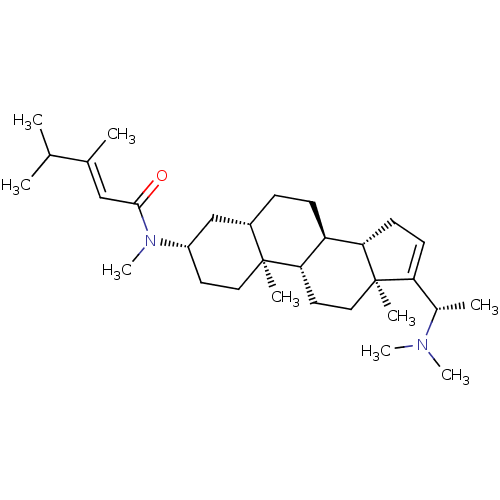 (1N-[14-(1-dimethylaminoethyl)-2,15-dimethyl-(1S,7S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421626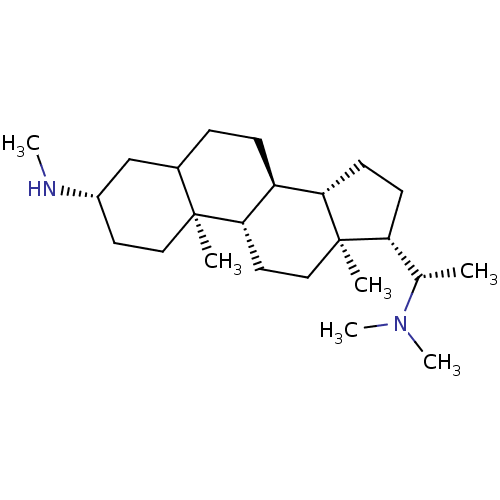 (CHEMBL139685) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135153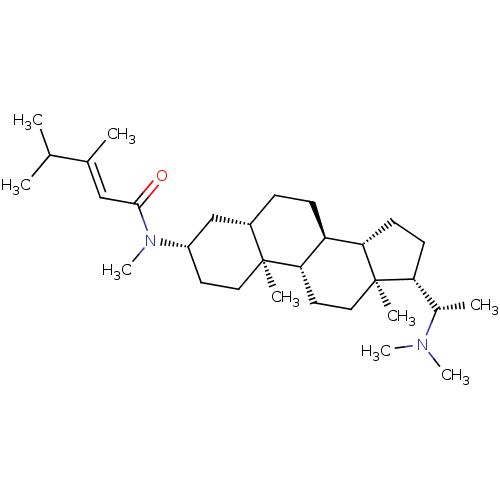 ((E)-3,4-Dimethyl-pent-2-enoic acid [(3S,5S,8R,9S,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421627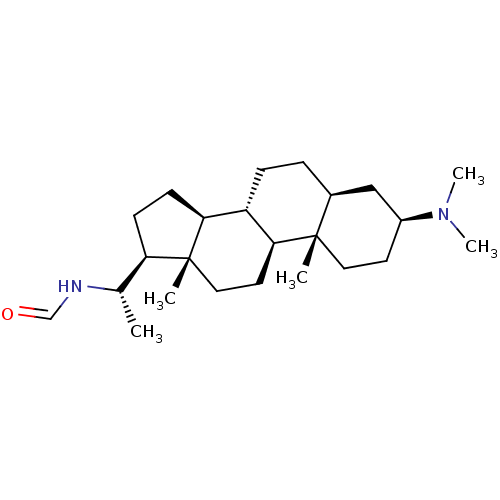 (CHEMBL136317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135158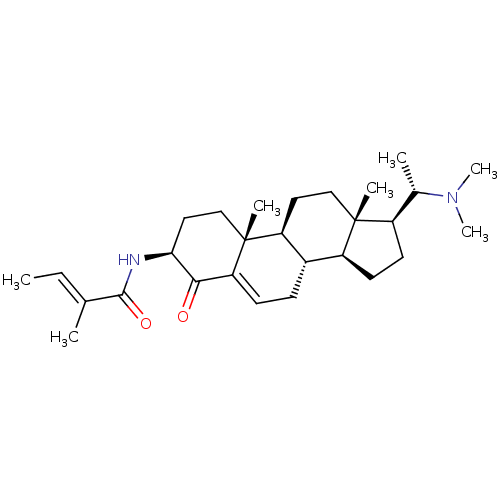 ((E)-2-Methyl-but-2-enoic acid [(3S,8S,9S,10R,13S,1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421630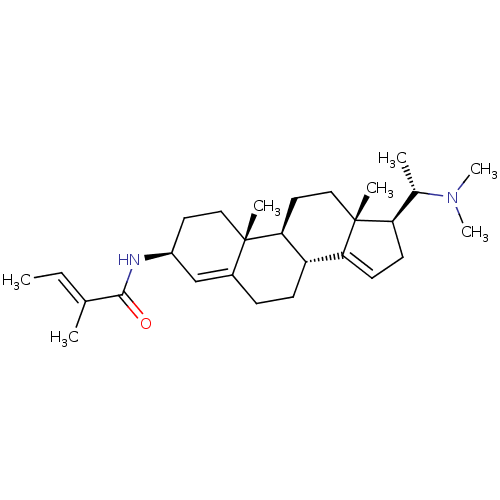 (CHEMBL136251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242345 (CHEMBL460447 | [(20S)-20-(N,N-dimethylamino)-3 bet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of horse BChE | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421625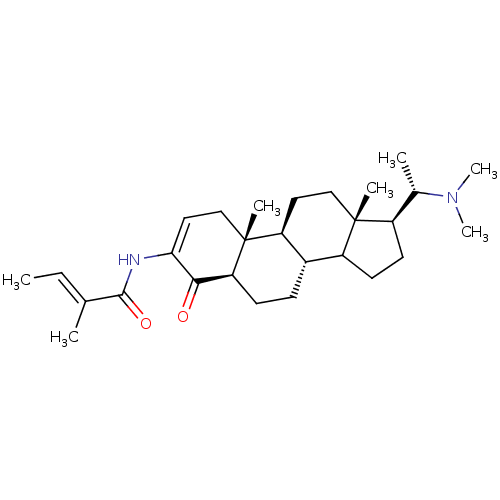 (CHEMBL136135) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421636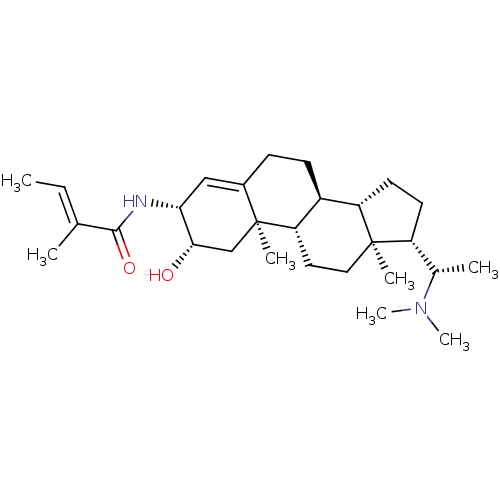 (CHEMBL139424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135159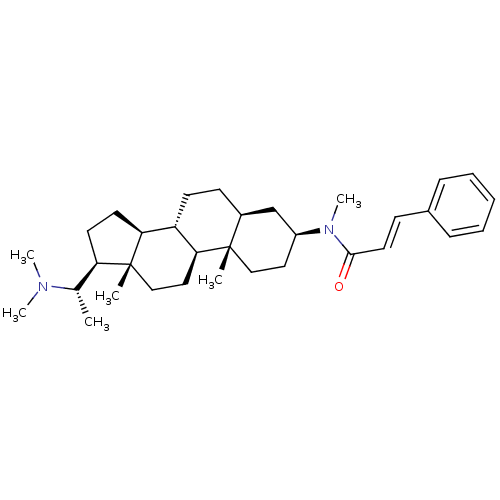 ((20S,2'E)-20-(N,N-dimethylamino)-3beta-(3'-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421631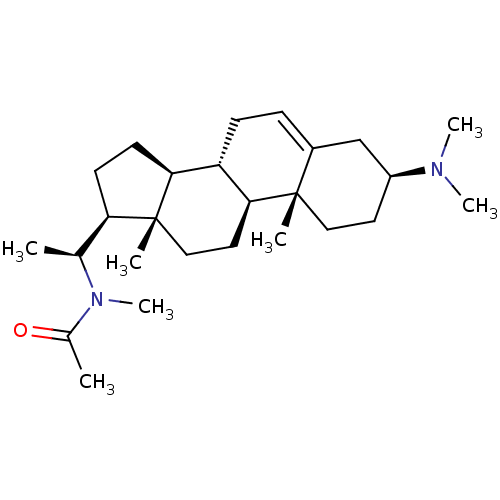 (CHEMBL136765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421634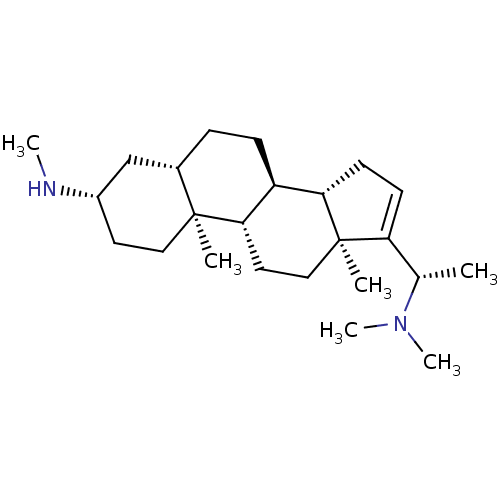 (CHEMBL137721) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM91719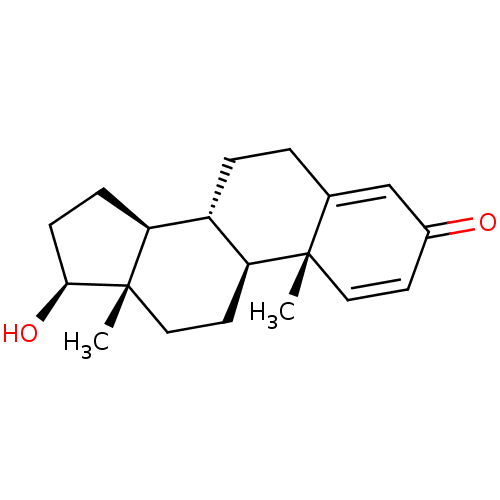 (1-Dehydrotestosterone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50242346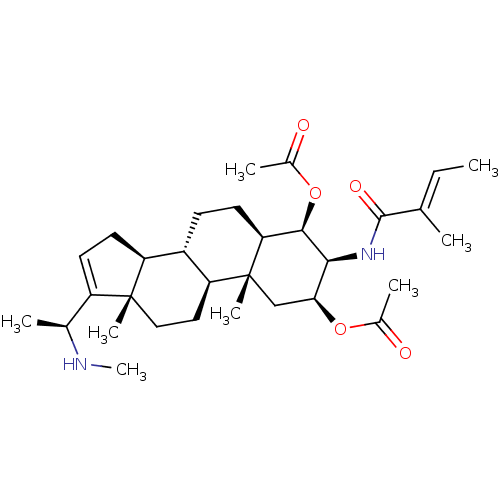 (CHEMBL500603 | [(20S)-20-(N-methylamino)-3beta-(ti...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of horse BChE | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50412078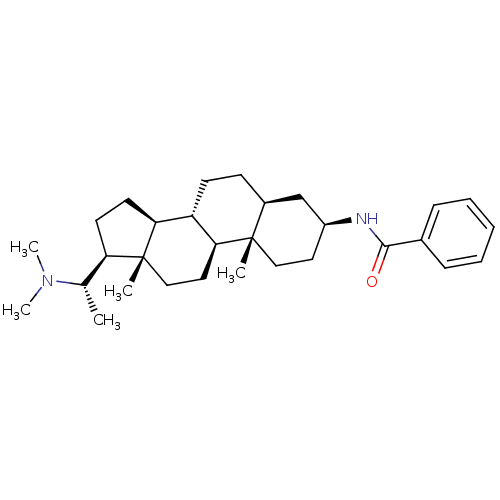 (EPIPACHYSAMINE D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421635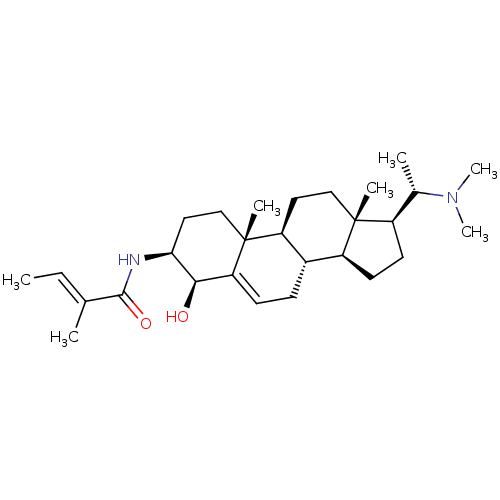 (CHEMBL2368067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50250634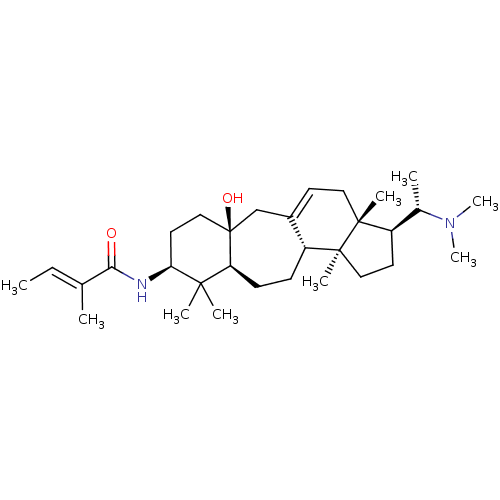 (CHEMBL466169 | [(20S)-20-(dimethylamino)-3-beta-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) | J Nat Prod 66: 739-42 (2003) Article DOI: 10.1021/np020446o BindingDB Entry DOI: 10.7270/Q2862G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421629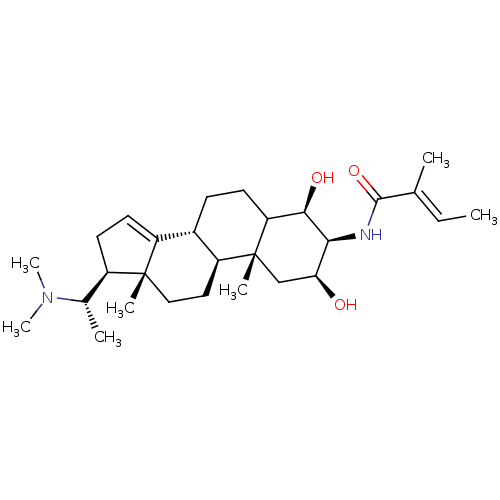 (CHEMBL337494) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421628 (CHEMBL344384) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50412080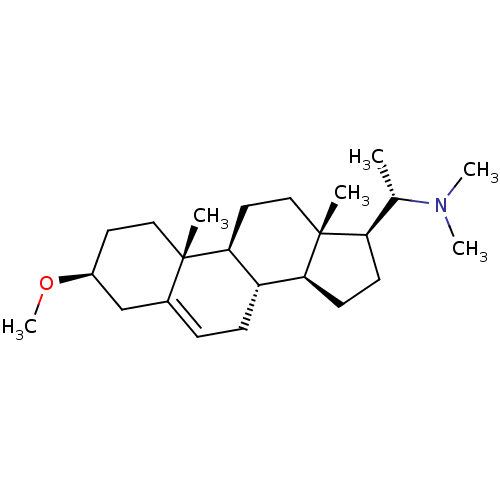 (CHEMBL342394) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242345 (CHEMBL460447 | [(20S)-20-(N,N-dimethylamino)-3 bet...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421623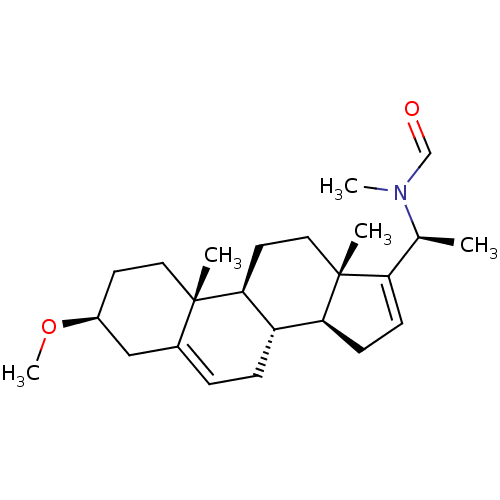 (CHEMBL140241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50242346 (CHEMBL500603 | [(20S)-20-(N-methylamino)-3beta-(ti...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tribhuvan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod 64: 842-4 (2001) BindingDB Entry DOI: 10.7270/Q20R9P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421632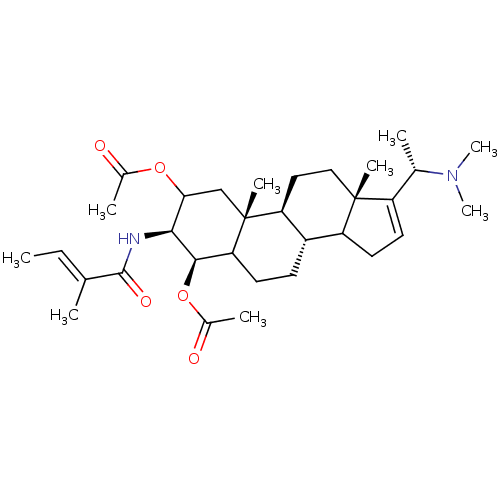 (CHEMBL342245) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50421624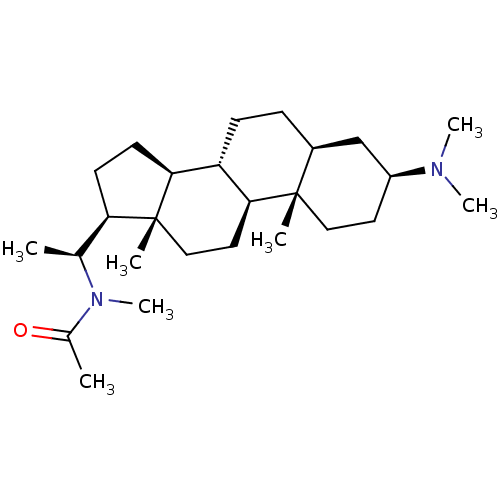 (CHEMBL136052) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8885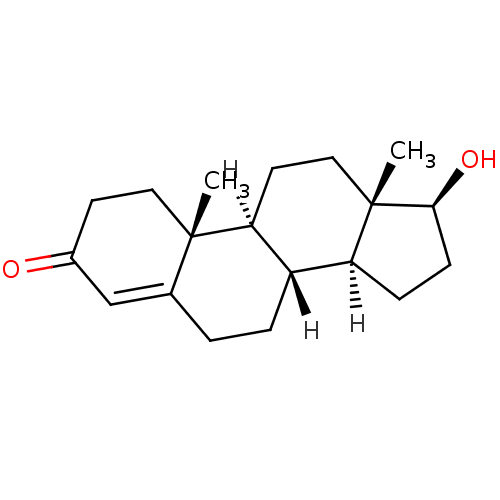 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50250635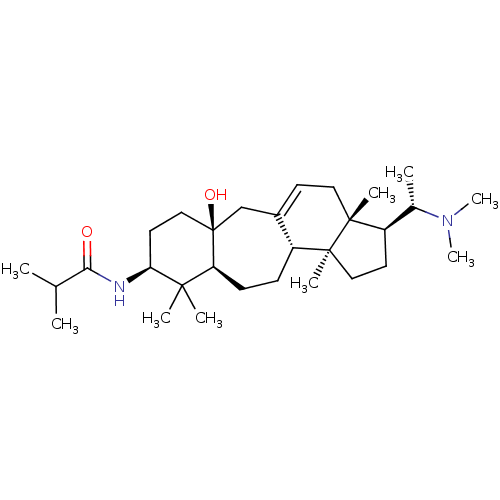 (CHEMBL517605 | [(20S)-20-(dimethylamino)-3-beta-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) | J Nat Prod 66: 739-42 (2003) Article DOI: 10.1021/np020446o BindingDB Entry DOI: 10.7270/Q2862G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM91722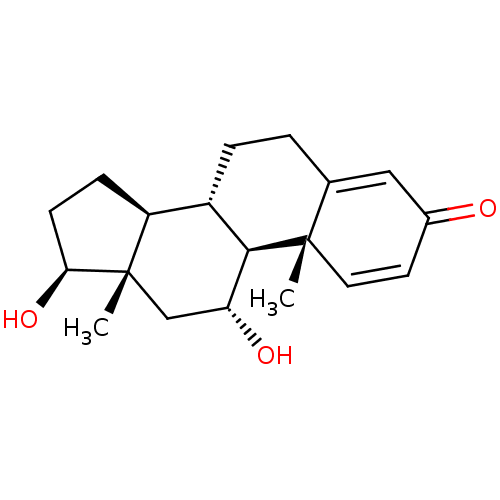 (11alpha-17beta-Dihydroxyandrost-1,4-dien-3-one, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jordan | Assay Description AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al. | J Enzyme Inhib Med Chem 24: 553-8 (2009) Article DOI: 10.1080/14756360802236393 BindingDB Entry DOI: 10.7270/Q20K274X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135156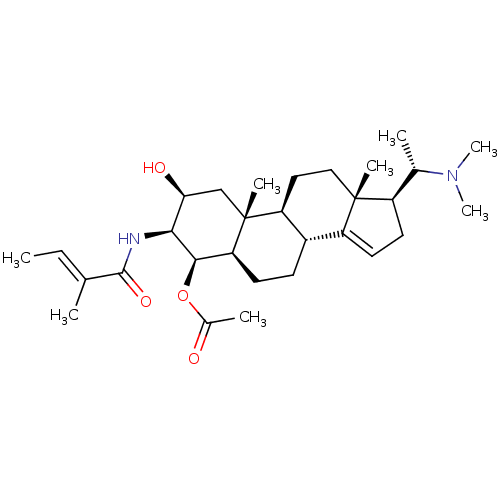 (Acetic acid (2S,3S,4R,5R,8R,9S,10R,13R,17S)-17-((S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50135152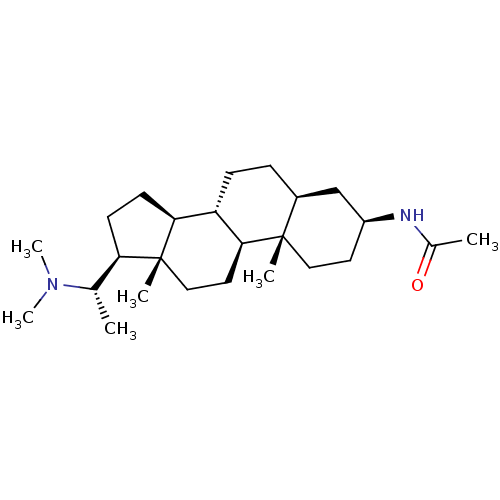 (CHEMBL422098 | N-[(3S,5S,8R,9S,10S,13S,14S,17S)-17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Compound was tested for the in silico inhibition of acetylcholinesterase | Bioorg Med Chem Lett 13: 4375-80 (2003) BindingDB Entry DOI: 10.7270/Q29C6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |